Abstract
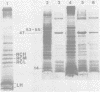
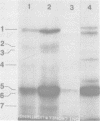
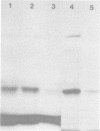
Cytoplasmic membranes (CM) prepared from both chemotrophic and phototrophic cells of Rhodopseudomonas sphaeroides possess penicillin-binding proteins (PBPs), as demonstrated by binding of [125]furazlocillin to isolated membranes, the subsequent separation of the constituent PBPs by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and their detection by autoradiography. The major PBP present in CM from R. sphaeroides corresponds in molecular weight to PBP-5, the predominant PBP present in CM of Escherichia coli. In contrast, the outer membrane of R. sphaeroides shows only low-level furazlocillin-binding activity on a per milligram of protein basis compared with chemotrophic CM. The intracytoplasmic membrane (ICM) derived from phototrophic cells contains less than 5% of the furazlocillin-binding activity of the CM. Based on the specific localization of PBPs in the CM, it is possible to provide quantitative estimates of the extent of CM present in preparations of ICM. This method demonstrates that highly purified preparations of ICM contain less than 5% CM. Additionally, the assay for PBPs demonstrates that during ICM remodeling, which occurs upon a shift from phototrophic to chemotrophic growth, there is no significant insertion of PBPs into the ICM over the first two generations after a shift to chemotrophic growth.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgardner D., Deal C., Kaplan S. Protein composition of Rhodopseudomonas sphaeroides outer membrane. J Bacteriol. 1980 Jul;143(1):265–273. doi: 10.1128/jb.143.1.265-273.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta G. A., Park J. T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981 Jan;145(1):333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- Brown A. E., Eiserling F. A., Lascelles J. Bacteriochlorophyll Synthesis and the Ultrastructure of Wild Type and Mutant Strains of Rhodopseudomonas spheroides. Plant Physiol. 1972 Dec;50(6):743–746. doi: 10.1104/pp.50.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. Competition between light and dark metabolism in Rhodospirillum rubrum. Arch Mikrobiol. 1955;22(2):195–203. doi: 10.1007/BF00409305. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- Cain B. D., Deal C. D., Fraley R. T., Kaplan S. In vivo intermembrane transfer of phospholipids in the photosynthetic bacterium Rhodopseudomonas sphaeroides. J Bacteriol. 1981 Mar;145(3):1154–1166. doi: 10.1128/jb.145.3.1154-1166.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. K., Kaplan S. The non-detergent solubilization and isolation of intracytoplasmic membrane polypeptides from Rhodopseudomonas sphaeroides. J Biol Chem. 1981 Jun 10;256(11):5901–5908. [PubMed] [Google Scholar]
- Connelly J. L., Jones O. T., Saunders V. A., Yates D. W. Kinetic and thermodynamic properties of membrane-bound cytochromes of aerobically and photosynthetically grown Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Apr 5;292(3):644–653. doi: 10.1016/0005-2728(73)90012-1. [DOI] [PubMed] [Google Scholar]
- Ding D. H., Kaplan S. Separation of inner and outer membranes of Rhodopseudomonas spheroides. Prep Biochem. 1976;6(1):61–79. doi: 10.1080/00327487608061599. [DOI] [PubMed] [Google Scholar]
- Firsow N. N., Drews G. Differentiation of the intracytoplasmic membrane of Rhodopseudomonas palustris induced by variations of oxygen partial pressure or light intensity. Arch Microbiol. 1977 Dec 15;115(3):299–306. doi: 10.1007/BF00446456. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Kaplan S. Isolation and fractionation of the photosynthetic membranous organelles from Rhodopseudomonas spheroides. J Bacteriol. 1971 Oct;108(1):465–473. doi: 10.1128/jb.108.1.465-473.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G. A., Richards W. R. Localization of photosynthetic membrane components in Rhodopseudomonas sphaeroides by a radioactive labeling procedure. Biochemistry. 1980 Oct 28;19(22):5104–5111. doi: 10.1021/bi00563a026. [DOI] [PubMed] [Google Scholar]
- Gorchein A. The relation between the pigment content of isolated chromatophores and that of the whole cell in Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Jul 2;170(1020):247–254. doi: 10.1098/rspb.1968.0036. [DOI] [PubMed] [Google Scholar]
- Guillotin J., Reiss-Husson F. Cytoplasmic and outer membranes separation in Rhodopseudomonas sphaeroides. Arch Microbiol. 1975 Nov 7;105(3):269–275. doi: 10.1007/BF00447146. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Anraku Y. Succinate dehydrogenase of Escherichia coli membrane vesicles. Activation and properties of the enzyme. J Biochem. 1974 Nov;76(5):959–966. [PubMed] [Google Scholar]
- King M. T., Drews G. The function and localization of ubiquinone in the NADH and succinate oxidase systems of Rhodopseudomonas palustris. Biochim Biophys Acta. 1973 May 30;305(2):230–248. doi: 10.1016/0005-2728(73)90172-2. [DOI] [PubMed] [Google Scholar]
- King M. T., Drews G. The respiratory electron transport system of heterotrophically-grown Rhodopseudomonas palustris. Arch Microbiol. 1975 Mar 10;102(3):219–231. doi: 10.1007/BF00428372. [DOI] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Cytochrome systems in dark-aerobically grown Rhodopseudomonas spheroides. Biochim Biophys Acta. 1966 Jun 8;120(2):274–281. doi: 10.1016/0926-6585(66)90347-5. [DOI] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Parks L. C., Niederman R. A. Membranes of Rhodopseudomonas sphaeroides. V. Identification of bacteriochlorophyll alpha-depleted cytoplasmic membrane in phototrophically grown cells. Biochim Biophys Acta. 1978 Jul 20;511(1):70–82. doi: 10.1016/0005-2736(78)90065-2. [DOI] [PubMed] [Google Scholar]
- Peters G. A., Cellarius R. A. Photosynthetic membrane development in Rhodopseudomonas spheroides. II. Correlation of pigment incorporation with morphological aspects of thylakoid formation. J Bioenerg. 1972 Aug;3(5):345–359. doi: 10.1007/BF01516074. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Shepherd W. D., Kaplan S. Effect of heat and 2-mercaptoethanol on intracytoplasmic membrane polypeptides of Rhodopseudomonas sphaeroides. J Bacteriol. 1978 Aug;135(2):656–667. doi: 10.1128/jb.135.2.656-667.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Takemoto J., Bachmann R. C. Orientation of chromatophores and spheroplast-derived membrane vesicles of Rhodopseudomonas sphaeroides: analysis by localization of enzyme activities. Arch Biochem Biophys. 1979 Jul;195(2):526–534. doi: 10.1016/0003-9861(79)90379-5. [DOI] [PubMed] [Google Scholar]
- Whale F. R., Jones O. T. The cytochrome system of heterotrophically-grown Rhodopseudomonas spheroides. Biochim Biophys Acta. 1970 Nov 3;223(1):146–157. doi: 10.1016/0005-2728(70)90139-8. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]