Abstract
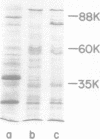
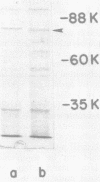
Three classes of mutants defective in the biosynthesis of the siderophore agrobactin were isolated from Agrobacterium tumefaciens A217 after N-methyl-N′-nitro-N-nitrosoguanidine mutagenesis. Class I mutants produced uniquely the catechol 2,3-dihydroxybenzoic acid, whereas classes II and III produced no detectable catechol. Class II differed from class III mutants in that exogenous 2,3-dihydroxybenzoic acid was utilized only by the former to synthesize agrobactin. Growth of strains B6 and A217, under iron starvation, led to enhanced production of several envelope proteins migrating in the 80,000-dalton range upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis. One mutant, defective in agrobactin iron utilization, lacked one of these proteins. This protein may represent a siderophore receptor or fragment or subunit thereof. With a single exception, all of the mutants obtained in this work were capable of initiating tumorous growth in sunflower plants and on carrot root disks, provided pTiB6806 was present. Comparison of the catechols produced by strain B6806 and its nononcogenic, Ti-plasmid-deficient derivative A217, indicated that the genes encoding agrobactin synthesis are not associated with the virulence plasmid of A. tumefaciens B6806. Analysis of gall tissue for agrobactin did not reveal the presence of this siderophore. Finally, citrate, an iron-carrier in plants, enhanced significantly the growth of the agrobactin-deficient mutants in a low-iron medium. These results suggest that the production of agrobactin in planta is not requisite to infection and that citrate may serve as an alternative carrier of iron for A. tumefaciens within the host.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. C., Chaney R. L. Effect of iron on the transport of citrate into the xylem of soybeans and tomatoes. Plant Physiol. 1971 Jun;47(6):836–840. doi: 10.1104/pp.47.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Evidence for diverse types of large plasmids in tumor-inducing strains of Agrobacterium. J Bacteriol. 1976 Apr;126(1):157–165. doi: 10.1128/jb.126.1.157-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Genetello C., Van Larebeke N., Holsters M., De Picker A., Van Montagu M., Schell J. Ti plasmids of Agrobacterium as conjugative plasmids. Nature. 1977 Feb 10;265(5594):561–563. doi: 10.1038/265561a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. A., Baldwin I. L., Riker A. J. Studies on Certain Physiological Characters of Phytomonas tumefaciens, Phytomonas rhizogenes and Bacillus radiobacter: Part II. J Bacteriol. 1934 Dec;28(6):597–618. doi: 10.1128/jb.28.6.597-618.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A., Manigault P., Tempé J. Transfer of virulence in vivo and in vitro in Agrobacterium. Nature. 1977 Feb 10;265(5594):560–561. doi: 10.1038/265560a0. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., de Jonge A. J., Schilperoort R. A., Rörsch A. An enrichment technique for auxotrophs of Agrobacterium tumefaciens using a combination of carbenicillin and lysozyme. J Gen Microbiol. 1975 Nov;91(1):177–182. doi: 10.1099/00221287-91-1-177. [DOI] [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lippincott J. A., Heberlein G. T. The quantitative determination of the infectivity of Agrobacterium tumefaciens. Am J Bot. 1965 Sep;52(8):856–863. [PubMed] [Google Scholar]
- Lippincott J. A., Lippincott B. B. Timing of events in crown-gall tumor development on Pinto bean leaves. Dev Biol. 1965 Oct;12(2):309–327. doi: 10.1016/0012-1606(65)90033-3. [DOI] [PubMed] [Google Scholar]
- Lippincott J. A., Webb J. H., Lippincott B. B. Auxotrophic mutation and infectivity of Agrobacterium tumefaciens. J Bacteriol. 1965 Oct;90(4):1155–1156. doi: 10.1128/jb.90.4.1155-1156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- McIntosh M. A., Pickett C. L., Chenault S. S., Earhart C. F. Suppression of iron uptake deficiency in Escherichia coli K-12 by loss of two major outer membrane proteins. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1106–1112. doi: 10.1016/0006-291x(78)91250-0. [DOI] [PubMed] [Google Scholar]
- Murphy T. P., Lean D. R., Nalewajko C. Blue-green algae: their excretion of iron-selective chelators enables them to dominate other algae. Science. 1976 May 28;192(4242):900–902. doi: 10.1126/science.818707. [DOI] [PubMed] [Google Scholar]
- Ong S. A., Peterson T., Neilands J. B. Agrobactin, a siderophore from Agrobacterium tumefaciens. J Biol Chem. 1979 Mar 25;254(6):1860–1865. [PubMed] [Google Scholar]
- Pollack J. R., Ames B. N., Neilands J. B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970 Nov;104(2):635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Tipton C. L., Klun J. A., Husted R. R., Pierson M. D. Cyclic hydroxamic acids and related compounds from maize. Isolation and characterization. Biochemistry. 1967 Sep;6(9):2866–2870. doi: 10.1021/bi00861a030. [DOI] [PubMed] [Google Scholar]
- Vervliet G., Holsters M., Teuchy H., Van Montagu M., Schell J. Characterization of different plaque-forming and defective temperate phages in Agrobacterium. J Gen Virol. 1975 Jan;26(1):33–48. doi: 10.1099/0022-1317-26-1-33. [DOI] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]