Abstract
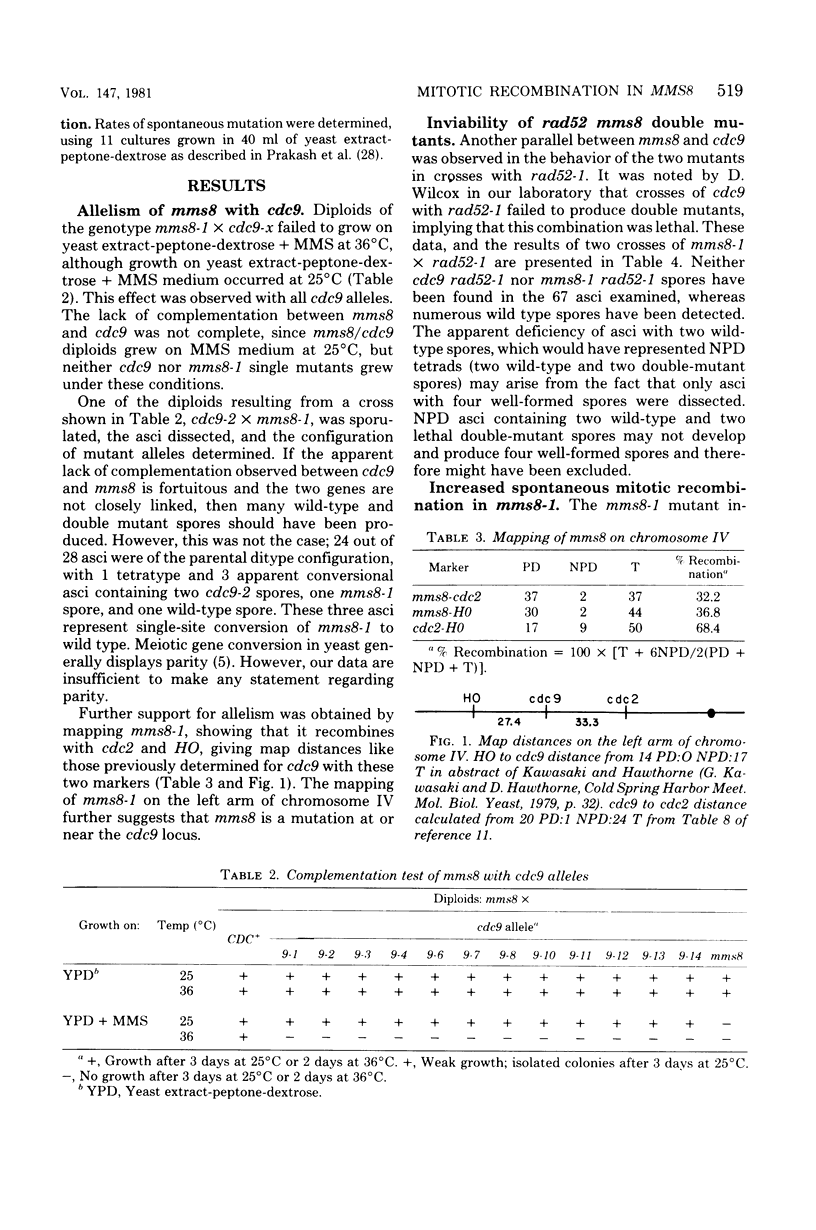
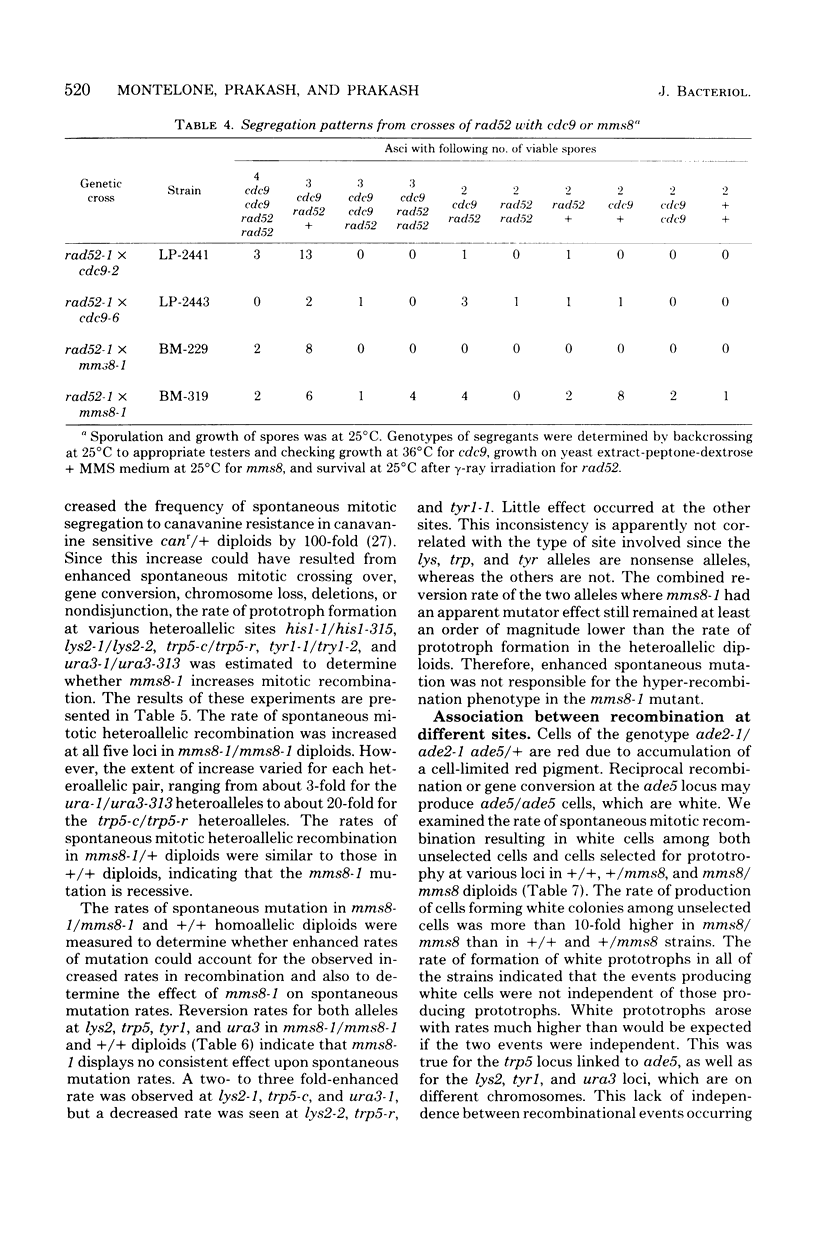
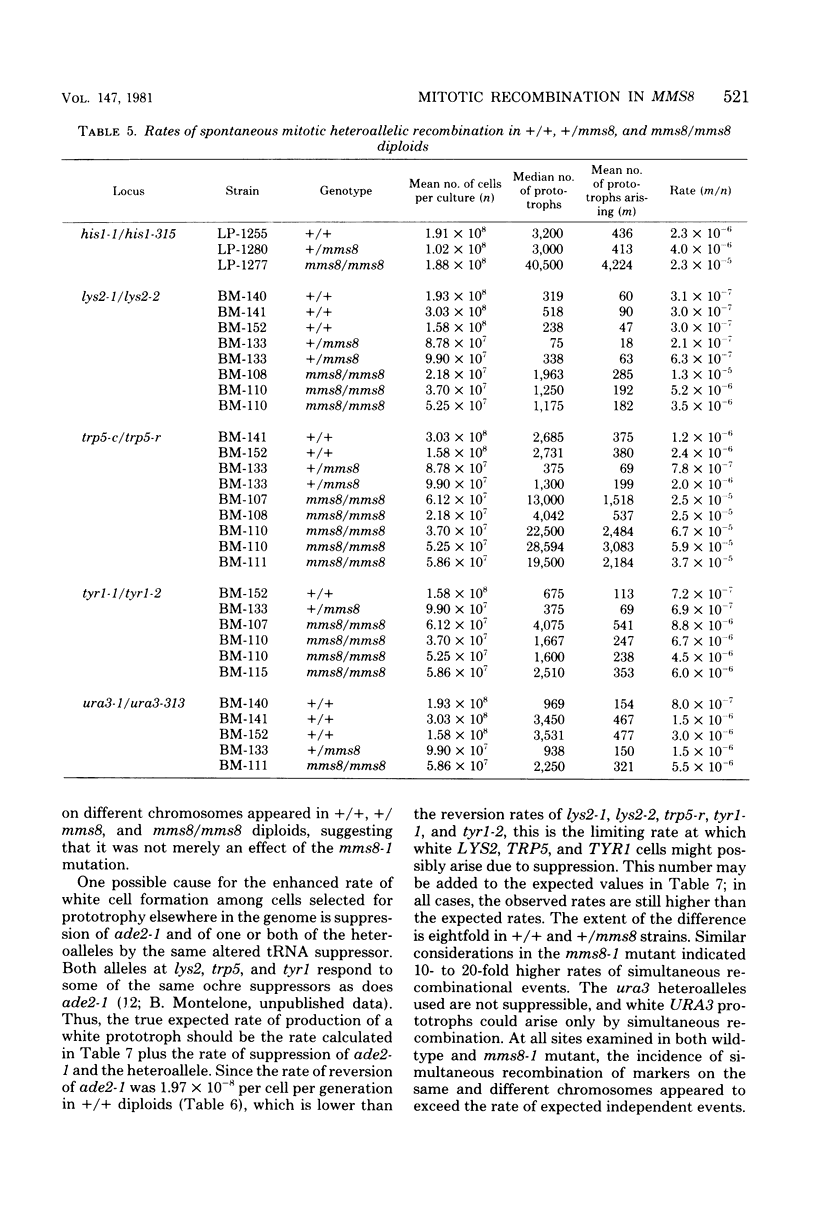
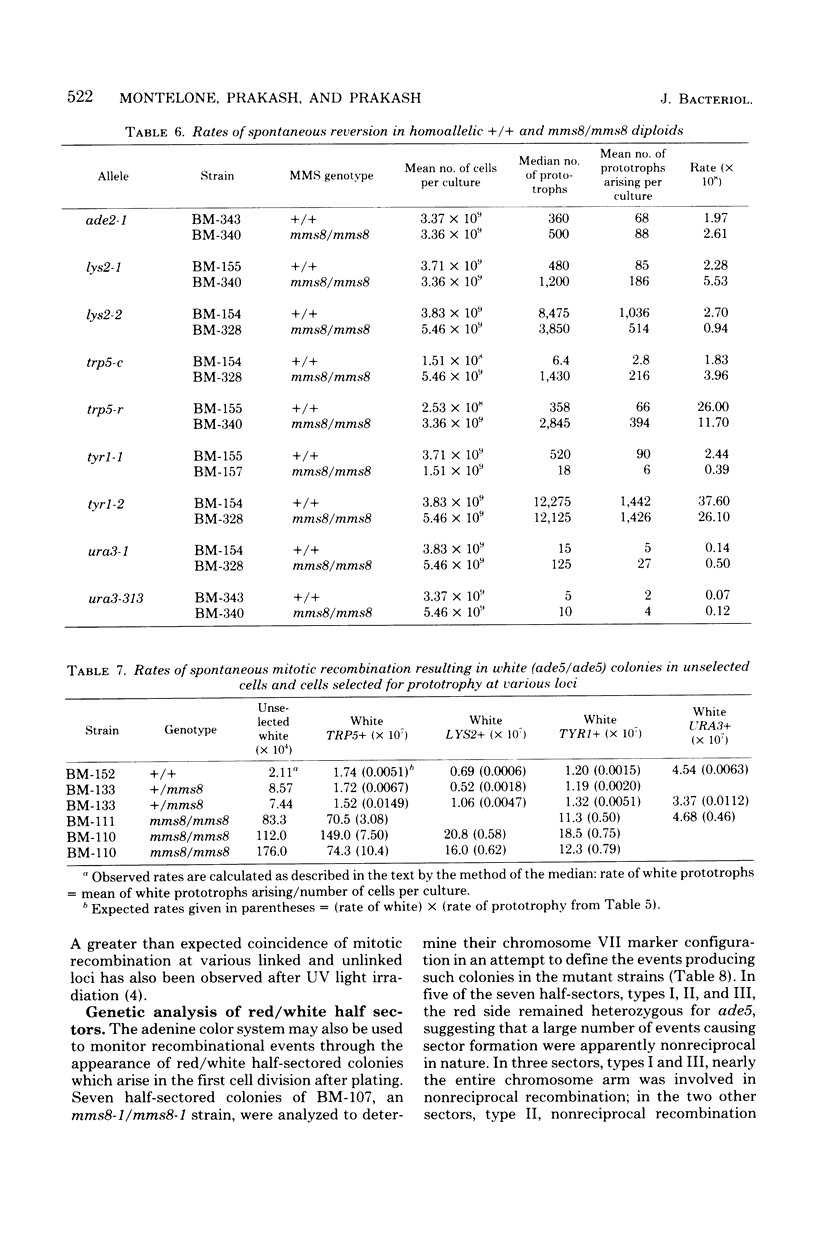
The methyl methane sulfonate (MMS)-sensitive mutation mms8-1 increases the rate of spontaneous mitotic intragenic recombination at five heteroallelic loci on three chromosomes. Complementation, segregation, and mapping studies indicate that mms8-1 is allelic to cdc9, known to be defective in deoxyribonucleic acid ligase. Both mms8-1 and cdc9 mutants are lethal in combination with the recombination-defective mutant rad52-1. Genetic analysis of spontaneous red/white sectors in an ade2-1/ade2-1 ade5/+ mms8-1/mms8-1 strain shows nonreciprocal recombinational events involving long chromosome segments. We also observe greater than expected rates of simultaneous recombination at loci on different chromosomes in both wild-type and mms8-1 mutants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boram W. R., Roman H. Recombination in Saccharomyces cerevisiae: a DNA repair mutation associated with elevated mitotic gene conversion. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2828–2832. doi: 10.1073/pnas.73.8.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S. Evidence that spontaneous mitotic recombination occurs at the two-strand stage. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4436–4440. doi: 10.1073/pnas.75.9.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOGEL S., HURST D. D. Coincidence relations between gene conversion and mitotic recombination in Saccharomyces. Genetics. 1963 Mar;48:321–328. doi: 10.1093/genetics/48.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre F. Induced intragenic recombination in yeast can occur during the G1 mitotic phase. Nature. 1978 Apr 27;272(5656):795–798. doi: 10.1038/272795a0. [DOI] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Game J. C., Johnston L. H., von Borstel R. C. Enhanced mitotic recombination in a ligase-defective mutant of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4589–4592. doi: 10.1073/pnas.76.9.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game J. C., Zamb T. J., Braun R. J., Resnick M., Roth R. M. The Role of Radiation (rad) Genes in Meiotic Recombination in Yeast. Genetics. 1980 Jan;94(1):51–68. doi: 10.1093/genetics/94.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golin J. E., Esposito M. S. Evidence for joint genic control of spontaneous mutation and genetic recombination during mitosis in Saccharomyces. Mol Gen Genet. 1977 Jan 18;150(2):127–135. doi: 10.1007/BF00695392. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne D. C., Leupold U. Suppressors in yeast. Curr Top Microbiol Immunol. 1974;64(0):1–47. doi: 10.1007/978-3-642-65848-8_1. [DOI] [PubMed] [Google Scholar]
- Johnston J. R. Genetic analysis of spontaneous half-sectored colonies of Saccharomyces cerevisiae. Genet Res. 1971 Oct;18(2):179–184. doi: 10.1017/s0016672300012581. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Nasmyth K. A. Saccharomyces cerevisiae cell cycle mutant cdc9 is defective in DNA ligase. Nature. 1978 Aug 31;274(5674):891–893. doi: 10.1038/274891a0. [DOI] [PubMed] [Google Scholar]
- Johnston L. H. The DNA repair capability of cdc9, the Saccharomyces cerevisiae mutant defective in DNA ligase. Mol Gen Genet. 1979 Feb 16;170(1):89–92. doi: 10.1007/BF00268583. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. A conditional lethal mutant of Escherichia coli K12 defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A. 1974 May;71(5):2048–2051. doi: 10.1073/pnas.71.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad E. B. Method for the isolation of Escherichia coli mutants with enhanced recombination between chromosomal duplications. J Bacteriol. 1977 Apr;130(1):167–172. doi: 10.1128/jb.130.1.167-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNEY T. R., MORTIMER R. K. ALLELIC MAPPING IN YEAST BY X-RAY-INDUCED MITOTIC REVERSION. Science. 1964 Feb 7;143(3606):581–583. doi: 10.1126/science.143.3606.581. [DOI] [PubMed] [Google Scholar]
- Maloney D. H., Fogel S. Mitotic recombination in yeast: isolation and characterization of mutants with enhanced spontaneous mitotic gene conversion rates. Genetics. 1980 Apr;94(4):825–839. doi: 10.1093/genetics/94.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Biological function for 6-methyladenine residues in the DNA of Escherichia coli K12. J Mol Biol. 1974 May 15;85(2):309–322. doi: 10.1016/0022-2836(74)90366-0. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive, radiation-sensitive mutant of Escherichia coli. II. DNA replication. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1195–1202. doi: 10.1073/pnas.64.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Prakash S. Isolation and characterization of MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics. 1977 May;86(1):33–55. doi: 10.1093/genetics/86.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Prakash L. Increased spontaneous mitotic segregation in MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics. 1977 Oct;87(2):229–236. doi: 10.1093/genetics/87.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenberg J. The relation of mitotic recombination to DNA replication in yeast pedigrees. Genetics. 1970 Oct;66(2):291–304. doi: 10.1093/genetics/66.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]



