Abstract
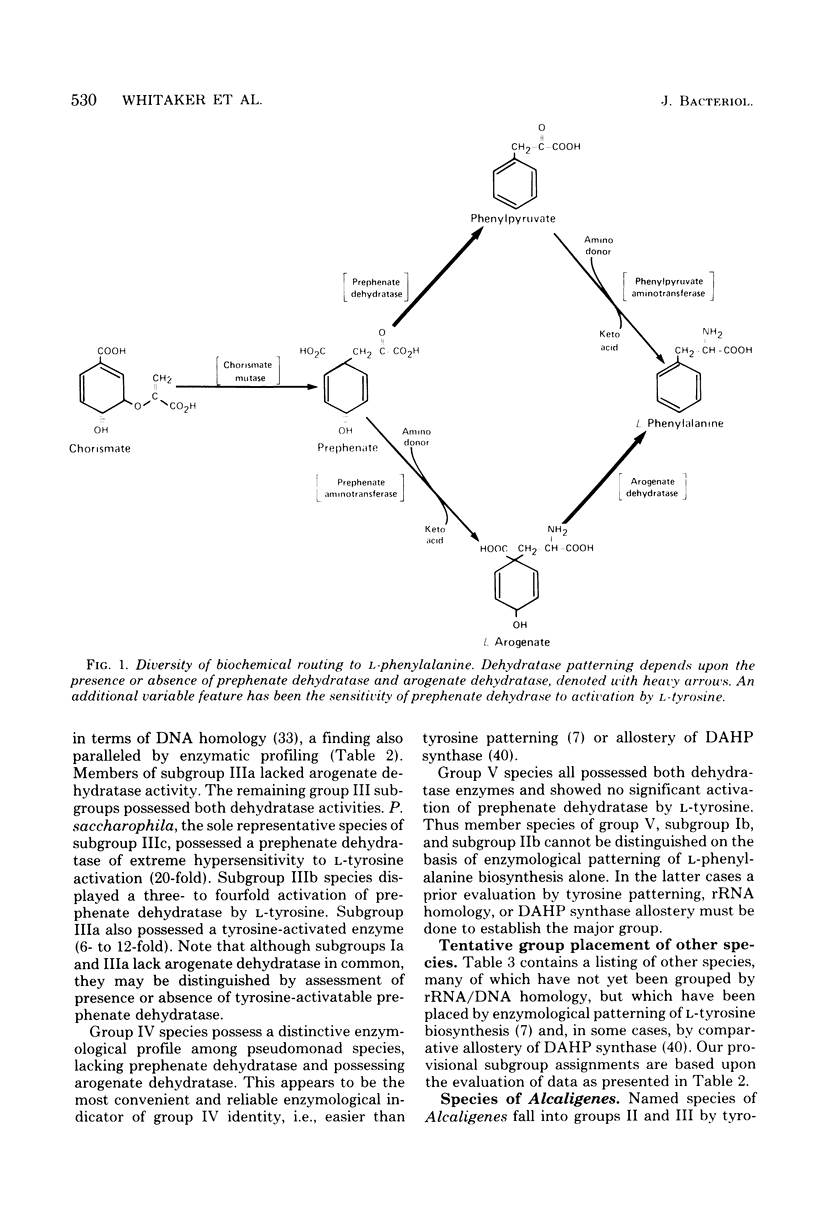
l-Tyrosine biosynthesis in nature has proven to be an exceedingly diverse gestalt of variable biochemical routing, cofactor specificity of pathway dehydrogenases, and regulation. A detailed analysis of this enzymological patterning of l-tyrosine biosynthesis formed a basis for the clean separation of five taxa among species currently named Pseudomonas, Xanthomonas, or Alcaligenes (Byng et al., J. Bacteriol. 144:247-257, 1980). These groupings paralleled taxa established independently by ribosomal ribonucleic acid/deoxyribonucleic acid (DNA) homology relationships. It was later found that the distinctive allosteric control of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase in group V, a group dominated by most named species of Xanthomonas (Whitaker et al., J. Bacteriol. 145:752-759, 1981), was the most striking and convenient criterion of group V identity. Diversity in the biochemical routing of l-phenylalanine biosynthesis and regulation was also found, and phenylalanine patterning is in fact the best single enzymatic indicator of group IV (Pseudomonas diminuta and Pseudomonas vesicularis) identity. Enzymological patterning of l-phenylalanine biosynthesis allowed discrimination of still finer groupings consistently paralleling that achieved by the criterion of DNA/DNA hybridization. Accordingly, the five ribosomal ribonucleic acid/DNA homology groups further separate into eight DNA homology subgroups and into nine subgroups based upon phenylalanine pathway enzyme profiling. (Although both fluorescent and nonfluorescent species of group I pseudomonads fall into a common DNA homology group, fluorescent species were distinct from nonfluorescent species in our analysis.) Hence, phenylalanine patterning data provide a relatively fine-tuned probe of hierarchical level. The combined application of these various enzymological characterizations, feasibly carried out in crude extracts, offers a comprehensive and reliable definition of 11 pseudomonad subgroups, 2 of them being represented by species of Alcaligenes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard R. W., Doudoroff M., Stanier R. Y., Mandel M. Taxonomy of the aerobic psuedomonads: Pseudomonas diminuta and P. vesiculare. J Gen Microbiol. 1968 Oct;53(3):349–361. doi: 10.1099/00221287-53-3-349. [DOI] [PubMed] [Google Scholar]
- Ballard R. W., Palleroni N. J., Doudoroff M., Stanier R. Y., Mandel M. Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola and P. caryophylli. J Gen Microbiol. 1970 Feb;60(2):199–214. doi: 10.1099/00221287-60-2-199. [DOI] [PubMed] [Google Scholar]
- Baptist J. N., Shaw C. R., Mandel M. Comparative zone electrophoresis of enzymes of Pseudomonas solanacearum and Pseudomonas cepacia. J Bacteriol. 1971 Nov;108(2):799–803. doi: 10.1128/jb.108.2.799-803.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann L., Baumann P. Studies of relationship among terrestrial Pseudomonas, Alcaligenes, and enterobacteria by an immunological comparison of glutamine synthetase. Arch Microbiol. 1978 Oct 4;119(1):25–30. doi: 10.1007/BF00407923. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byng G. S., Whitaker R. J., Gherna R. L., Jensen R. A. Variable enzymological patterning in tyrosine biosynthesis as a means of determining natural relatedness among the Pseudomonadaceae. J Bacteriol. 1980 Oct;144(1):247–257. doi: 10.1128/jb.144.1.247-257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byng G. S., Whitaker R. J., Shapiro C. L., Jensen R. A. The aromatic amino acid pathway branches at L-arogenate in Euglena gracilis. Mol Cell Biol. 1981 May;1(5):426–438. doi: 10.1128/mcb.1.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. Channel-shuttle mechanism for the regulation of phenylalanine and tyrosine synthesis at a metabolic branch point in Pseudomonas aeruginosa. J Bacteriol. 1973 Jan;113(1):241–251. doi: 10.1128/jb.113.1.241-251.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel A. M., Bowen J. R., Jensen R. A. Arogenate (pretyrosine) is an obligatory intermediate of L-tyrosine biosynthesis: confirmation in a microbial mutant. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1270–1273. doi: 10.1073/pnas.77.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOWAY B. W. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol. 1955 Dec;13(3):572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H., Stenmark S. L. Allosteric inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase by tyrosine, tryptophan and phenylpyruvate in Pseudomonas aeruginosa. Biochim Biophys Acta. 1973 Jan 12;293(1):256–268. doi: 10.1016/0005-2744(73)90398-7. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Pierson D. L. Evolutionary implications of different types of microbial enzymology for L-tyrosine biosynthesis. Nature. 1975 Apr 24;254(5502):667–671. doi: 10.1038/254667a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Stenmark S. L. Comparative allostery of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthetase as a molecular basis for classification. J Bacteriol. 1970 Mar;101(3):763–769. doi: 10.1128/jb.101.3.763-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LéJohn H. B. Enzyme regulation, lysine pathways and cell wall structures as indicators of major lines of evolution in fungi. Nature. 1971 May 21;231(5299):164–168. doi: 10.1038/231164a0. [DOI] [PubMed] [Google Scholar]
- Palleroni N. J., Ballard R. W., Ralston E., Doudoroff M. Deoxyribonucleic acid homologies among some Pseudomonas species. J Bacteriol. 1972 Apr;110(1):1–11. doi: 10.1128/jb.110.1.1-11.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleroni N. J., Doudoroff M. Phenotypic characterization and deoxyribonucleic acid homologies of Pseudomonas solanacearum. J Bacteriol. 1971 Sep;107(3):690–696. doi: 10.1128/jb.107.3.690-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleroni N. J., Doudoroff M., Stanier R. Y., Solánes R. E., Mandel M. Taxonomy of the aerobic pseudomonads: the properties of the Pseudomonas stutzeri group. J Gen Microbiol. 1970 Feb;60(2):215–231. doi: 10.1099/00221287-60-2-215. [DOI] [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Patel N., Stenmark-Cox S. L., Jensen R. A. Enzymological basis of reluctant auxotrophy for phenylalanine and tyrosine in Pseudomonas aeruginosa. J Biol Chem. 1978 May 10;253(9):2972–2978. [PubMed] [Google Scholar]
- Ralston E., Palleroni N. J., Doudoroff M. Deoxyribonucleic acid homologies of some so-called "Hydrogenomonas" species. J Bacteriol. 1972 Jan;109(1):465–466. doi: 10.1128/jb.109.1.465-466.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro C. L., Jensen R. A., Wilson K. A., Bowen J. R. An assay for activity of arogenate dehydratase base upon the selective oxidation of arogenate. Anal Biochem. 1981 Jan 1;110(1):27–30. doi: 10.1016/0003-2697(81)90106-8. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stenmark-Cox S., Jensen R. A. Prephenate dehydrogenase from Pseudomonas aeruginosa is a regulated component of the channel-shuttle mechanism controlling tyrosine-phenylalanine synthesis. Arch Biochem Biophys. 1975 Apr;167(2):540–546. doi: 10.1016/0003-9861(75)90497-x. [DOI] [PubMed] [Google Scholar]
- Stenmark S. L., Pierson D. L., Jensen R. A., Glover G. I. Blue-green bacteria synthesise L-tyrosine by the pretyrosine pathway. Nature. 1974 Feb 1;247(5439):290–292. doi: 10.1038/247290a0. [DOI] [PubMed] [Google Scholar]
- Whitaker R. J., Byng G. S., Gherna R. L., Jensen R. A. Comparative allostery of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase as an indicator of taxonomic relatedness in pseudomonad genera. J Bacteriol. 1981 Feb;145(2):752–759. doi: 10.1128/jb.145.2.752-759.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



