Abstract
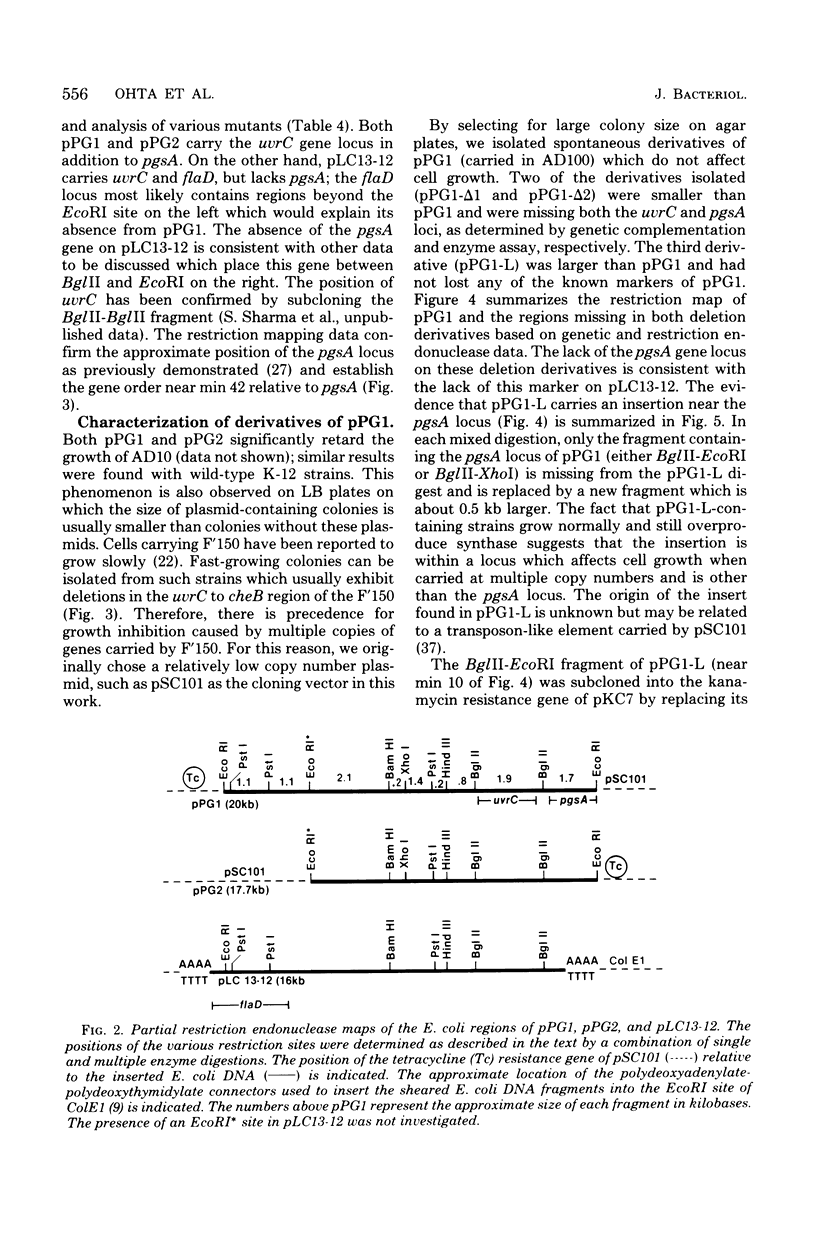
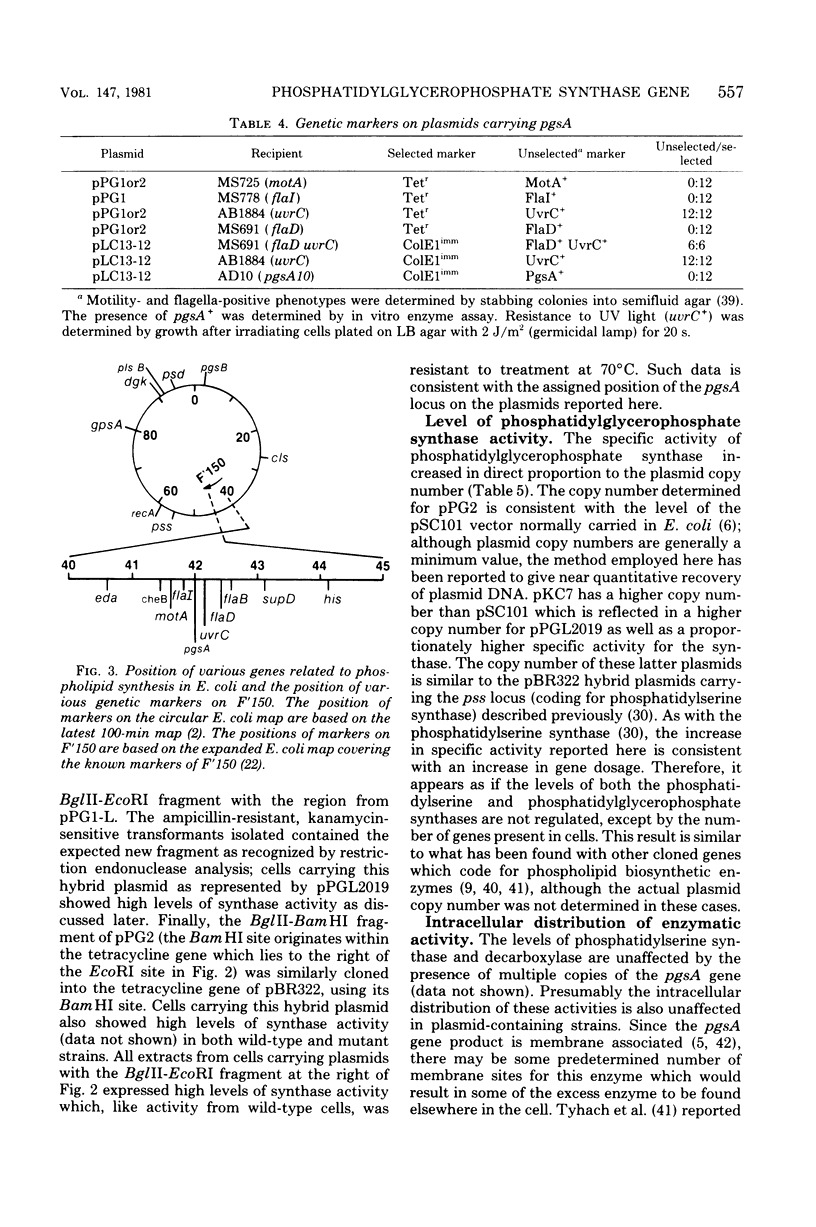
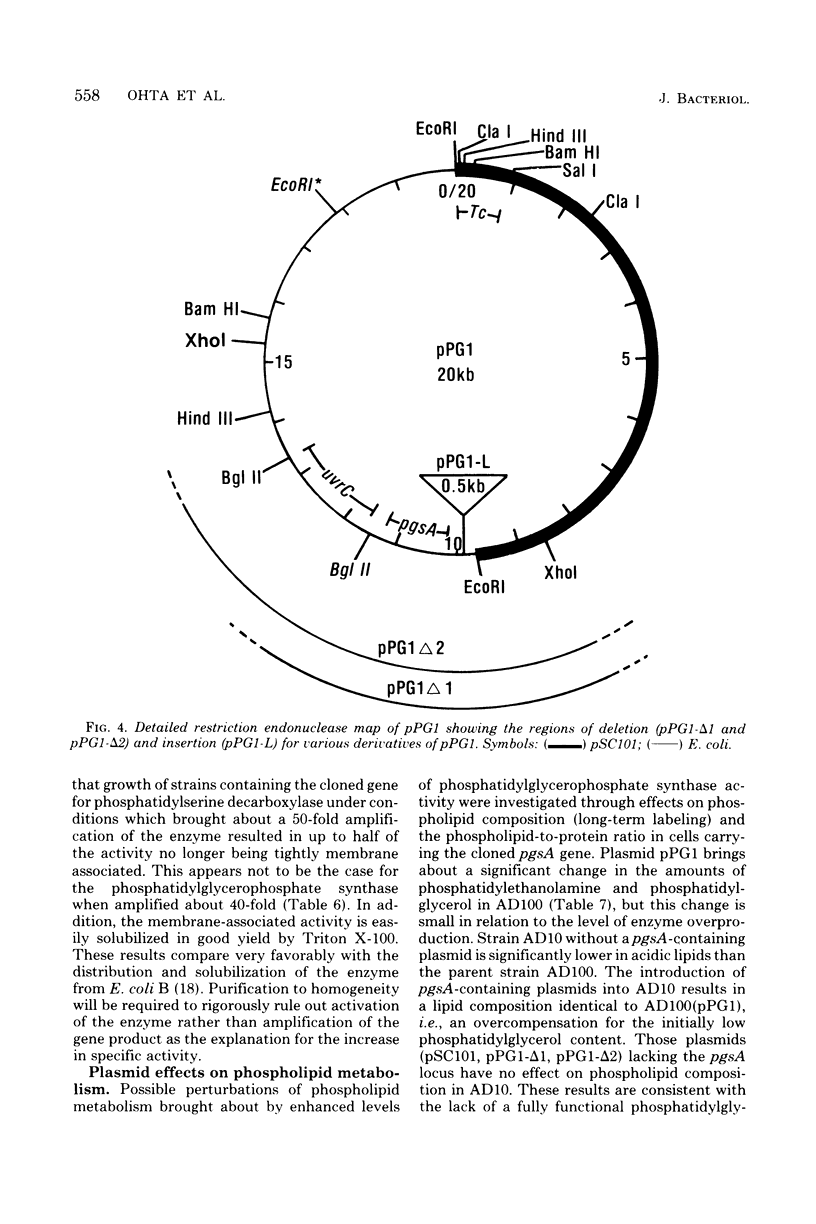
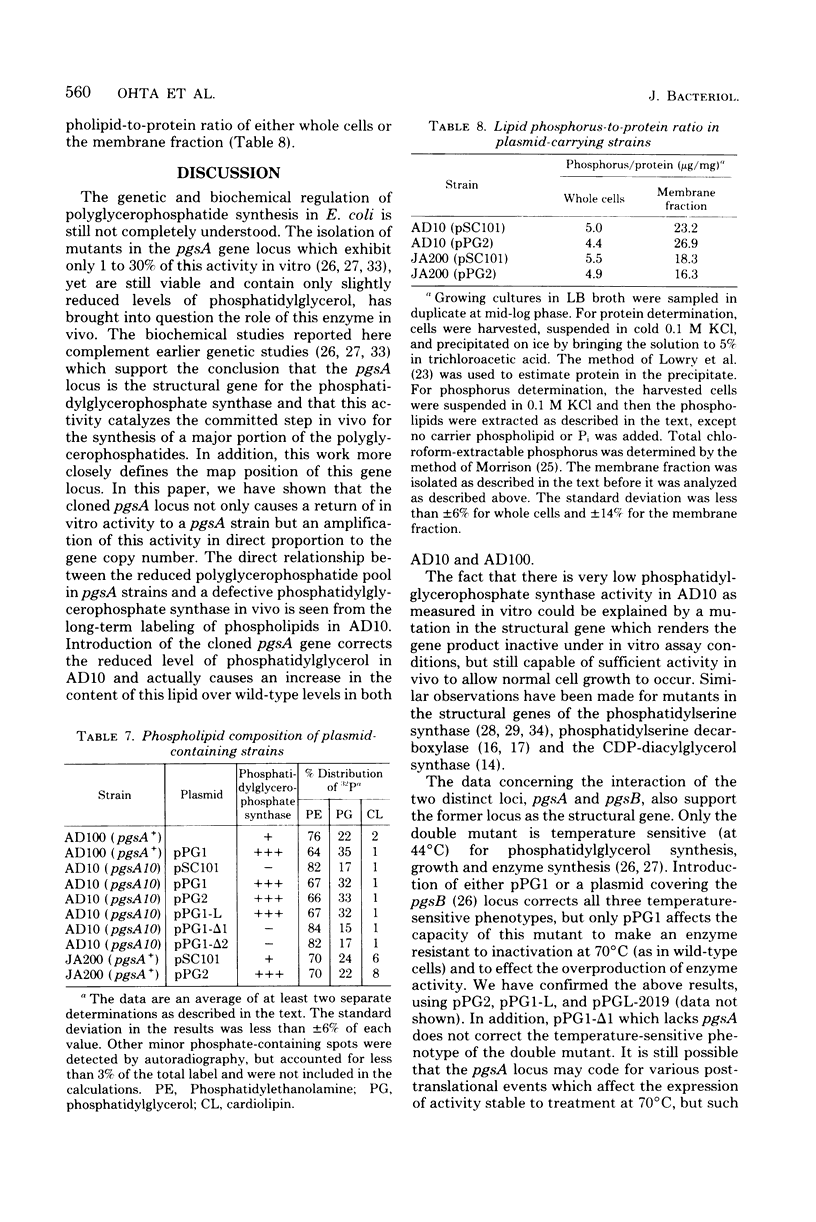
The structural gene (pgsA) for the CDP-diacylglycerol:sn-glycero-3-phosphate phosphatidyltransferase (EC 2.7.8.5, phosphatidylglycerophosphate synthase) from Escherichia coli has been cloned, using pSC101 as the vector. The resulting hybrid plasmids not only correct the lack of in vitro synthase activity in pgsA strains but also cause an amplification (6- to 40-fold over wild-type levels) in enzymatic activity in direct proportion to the copy number of the plasmids found in vivo. The cloned gene also corrects the abnormally low level of polyglycerophosphatides found in pgsA strains and actually increases the level of phosphatidylglycerol to above that normally found in E. coli. The degree of alteration in phospholipid composition brought about by these hybrid plasmids is not of the order expected if fluctuations in enzyme levels in vivo were an important regulatory mechanism in phospholipid metabolism. The isolated hybrid plasmids have been mapped by restriction endonuclease analysis. The presence and location of other genetic markers have also been established. The above data, along with analysis of deletion derivatives of these plasmids and subcloning of appropriate restriction fragments, have established the position of the pgsA locus on the hybrid plasmids. From this data, the position of the pgsA locus has been determined to le between flaI and uvrC on the E. coli genetic map.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello F., Timmis K., Cohen S. N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976 Jan 29;259(5541):285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Dowhan W. Phosphatidylserine synthase from Escherichia coli. The role of Triton X-100 in catalysis. J Biol Chem. 1979 Sep 10;254(17):8391–8397. [PubMed] [Google Scholar]
- Clark D., Lightner V., Edgar R., Modrich P., Cronan J. E., Jr, Bell R. M. Regulation of phospholipid biosynthesis in Escherichia coli. Cloning of the structural gene for the biosynthetic sn-glycerol-3-phosphate dehydrogenase. J Biol Chem. 1980 Jan 25;255(2):714–717. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowhan W., Wickner W. T., Kennedy E. P. Purification and properties of phosphatidylserine decarboxylase from Escherichia coli. J Biol Chem. 1974 May 25;249(10):3079–3084. [PubMed] [Google Scholar]
- Ganong B. R., Leonard J. M., Raetz C. R. Phosphatidic acid accumulation in the membranes of Escherichia coli mutants defective in CDP-diglyceride synthetase. J Biol Chem. 1980 Feb 25;255(4):1623–1629. [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Conditional lethal phosphatidylserine decarboxylase mutants of Escherichia coli. Mapping of the structural gene for phosphatidylserine decarboxylase. Mol Gen Genet. 1976 Nov 17;148(3):271–279. doi: 10.1007/BF00332901. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Phospholipid composition and membrane function in phosphatidylserine decarboxylase mutants of Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8213–8220. [PubMed] [Google Scholar]
- Hirabayashi T., Larson T. J., Dowhan W. Membrane-associated phosphatidylglycerophosphate synthetase from Escherichia coli: purification by substrate affinity chromatography on cytidine 5'-diphospho-1,2-diacyl-sn-glycerol sepharose. Biochemistry. 1976 Nov 30;15(24):5205–5211. doi: 10.1021/bi00669a002. [DOI] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson T. J., Dowhan W. Ribosomal-associated phosphatidylserine synthetase from Escherichia coli: purification by substrate-specific elution from phosphocellulose using cytidine 5'-diphospho-1,2-diacyl-sn-glycerol. Biochemistry. 1976 Nov 30;15(24):5212–5218. doi: 10.1021/bi00669a003. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R. A FAST, SIMPLE AND RELIABLE METHOD FOR THE MICRODETERMINATION OF PHOSPHORUS IN BIOLOGICAL MATERIALS. Anal Biochem. 1964 Feb;7:218–224. doi: 10.1016/0003-2697(64)90231-3. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Bulawa C. E., Raetz C. R. Two interacting mutations causing temperature-sensitive phosphatidylglycerol synthesis in Escherichia coli membranes. J Bacteriol. 1981 Jan;145(1):113–121. doi: 10.1128/jb.145.1.113-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J Biol Chem. 1979 Aug 25;254(16):7837–7844. [PubMed] [Google Scholar]
- Ohta A., Waggoner K., Louie K., Dowhan W. Cloning of genes involved in membrane lipid synthesis. Effects of amplification of phosphatidylserine synthase in Escherichia coli. J Biol Chem. 1981 Mar 10;256(5):2219–2225. [PubMed] [Google Scholar]
- Ota A., Shibuya I., Maruo B., Ishinaga M., Kito M. An extremely labile phosphatidylserine synthetase of an Escherichia coli mutant with the temperature-sensitive formation of phosphatidylethanolamine. Biochim Biophys Acta. 1974 Jun 26;348(3):449–454. [PubMed] [Google Scholar]
- Pluschke G., Hirota Y., Overath P. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J Biol Chem. 1978 Jul 25;253(14):5048–5055. [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Silverman M., Matsumura P., Hilmen M., Simon M. Characterization of lambda Escherichia coli hybrids carrying chemotaxis genes. J Bacteriol. 1977 May;130(2):877–887. doi: 10.1128/jb.130.2.877-887.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Genetic analysis of flagellar mutants in Escherichia coli. J Bacteriol. 1973 Jan;113(1):105–113. doi: 10.1128/jb.113.1.105-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyhach R. J., Hawrot E., Satre M., Kennedy E. P. Increased synthesis of phosphatidylserine decarboxylase in a strain of Escherichia coli bearing a hybrid plasmid. Altered association of enzyme with the membrane. J Biol Chem. 1979 Feb 10;254(3):627–633. [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]