Abstract
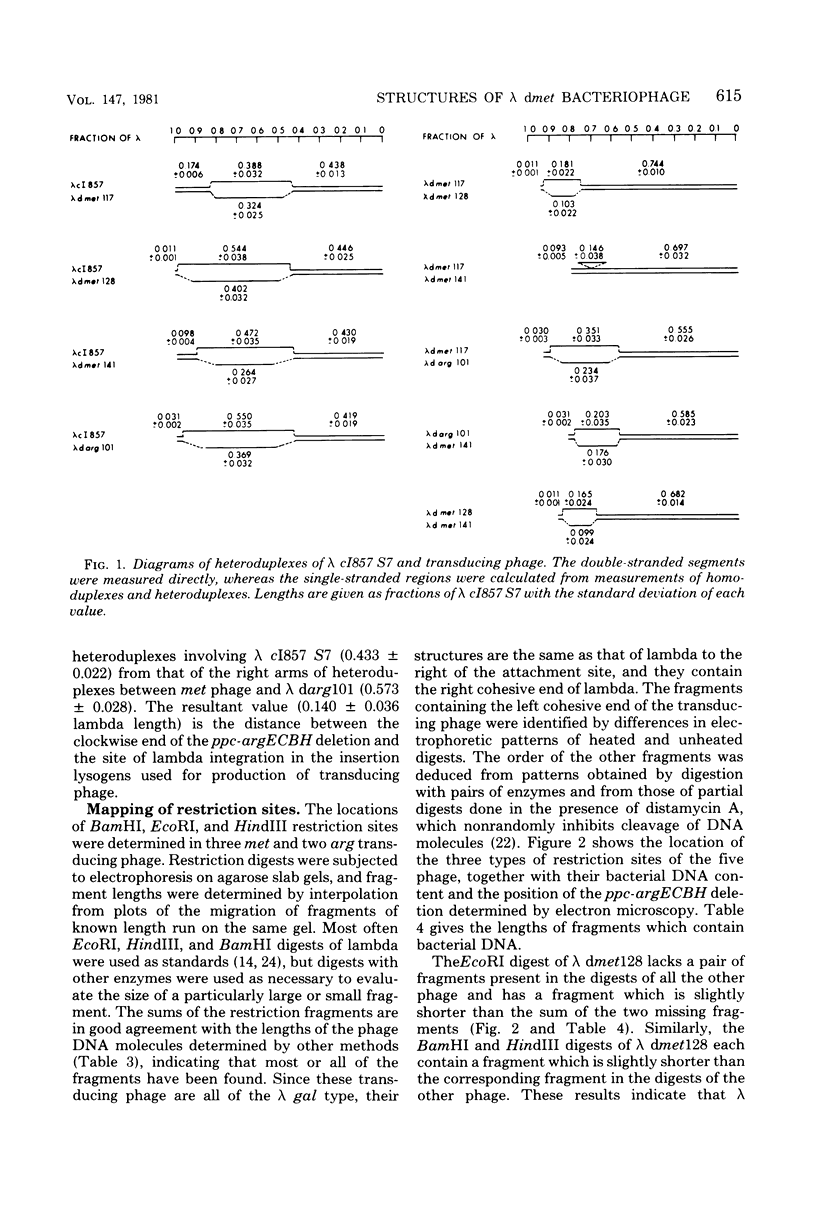
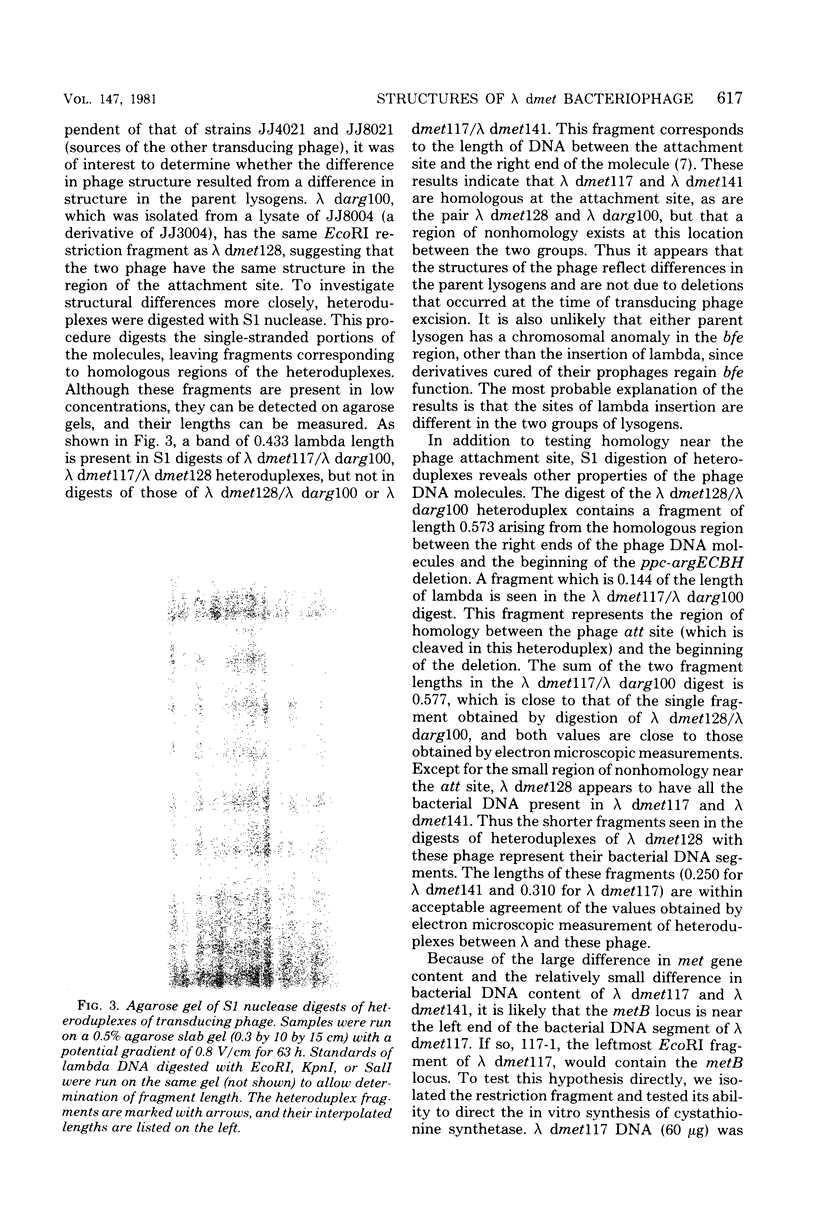
The structures of several lambda dmet and related lambda darg transducing phage were studied by restriction fragment mapping and electron microscopic measurements of homoduplexes and heteroduplexes. A new transducing phage (lambda dmet141), in which metF is the only functional gene of the cluster, was isolated. In contrast, lambda dmet117, which expresses the entire metBJLF cluster, has only 3 kilobases more bacterial deoxyribonucleic acid (DNA) than lambda dmet141. An EcoRI restriction fragment of lambda dmet117, which carries the leftmost 6 kilobases of the bacterial DNA insert, was isolated and shown to contain a functional copy of metB. Small structural differences at the attachment sites of some of the phage were shown to result from different sites of lambda integration in the two parent insertion lysogens.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M. Preparation of guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) from Escherichia coli ribosomes. Anal Biochem. 1974 Jan;57(1):100–107. doi: 10.1016/0003-2697(74)90056-6. [DOI] [PubMed] [Google Scholar]
- Cossart P., Katinka M., Yaniv M., Saint Girons I., Cohen G. N. Construction and expression of a hybrid plasmid containing the Escherichia coli thrA and thrB genes. Mol Gen Genet. 1979 Aug;175(1):39–44. doi: 10.1007/BF00267853. [DOI] [PubMed] [Google Scholar]
- Coward J. K., Chello P. L., Cashmore A. R., Parameswaran K. N., DeAngelis L. M., Bertino J. R. 5-methyl-5,6,7,8-tetrahydropteroyl oligo-gamma-L-glutamates: synthesis and kinetic studies with methionine synthetase from bovine brain. Biochemistry. 1975 Apr 8;14(7):1548–1552. doi: 10.1021/bi00678a032. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Glansdorff N. Studies on the bipolar argECBH operon of E. coli: characterization of restriction endonuclease fragments obtained from gammadargECBH transducing phages and a ColE1 argECBH plasmid. Mol Gen Genet. 1977 Mar 7;151(2):161–168. doi: 10.1007/BF00338690. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Sibilli-Weill L., Cohen G. N. Subunit structure of the methionine-repressible aspartokinase II--homoserine dehydrogenase II from Escherichia coli K12. Eur J Biochem. 1977 Jun 1;76(1):1–6. doi: 10.1111/j.1432-1033.1977.tb11563.x. [DOI] [PubMed] [Google Scholar]
- Echols H., Murialdo H. Genetic map of bacteriophage lambda. Microbiol Rev. 1978 Sep;42(3):577–591. doi: 10.1128/mr.42.3.577-591.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers D., Cunin R., Glansdorff N. Control regions within the argECBH gene cluster of Escherichia coli K12. Mol Gen Genet. 1972;117(4):349–366. doi: 10.1007/BF00333028. [DOI] [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Synthesis of the succinic ester of homoserine, a new intermediate in the bacterial biosynthesis of methionine. Biochemistry. 1965 Jul;4(7):1370–1375. doi: 10.1021/bi00883a022. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C. Improved procedure for synthesis of radioactive O-succinyl-L-homoserine. Anal Biochem. 1977 Mar;78(1):182–187. doi: 10.1016/0003-2697(77)90022-7. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Radovich C. Role of methionine in the regulation of serine hydroxymethyltransferase in Eschericia coli. J Bacteriol. 1975 Oct;124(1):269–278. doi: 10.1128/jb.124.1.269-278.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty D. M., Scheif R. F. Location in bacteriophage lamdba DNA of cleavage sites of the site-specific endonuclease from Bacillus amyloliquefaciens H. J Virol. 1976 May;18(2):659–663. doi: 10.1128/jvi.18.2.659-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway C. T., Greene R. C., Su C. H. Regulation of S-adenosylmethionine synthetase in Escherichia coli. J Bacteriol. 1970 Nov;104(2):734–747. doi: 10.1128/jb.104.2.734-747.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Greene R. C., Krueger J. H. Isolation and characterization of specialized lambda transducing bacteriophage carrying the metBJF methionine gene cluster. J Bacteriol. 1977 Sep;131(3):795–800. doi: 10.1128/jb.131.3.795-800.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad B., Kirschbaum J., Austin S. Isolation and characterization of phi80 transducing bacteriophage for a ribonucleic acid polymerase gene. J Bacteriol. 1973 Nov;116(2):511–516. doi: 10.1128/jb.116.2.511-516.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. H., Johnson J. R., Greene R. C. In vitro synthesis of cystathionine gamma-synthetase in Escherichia coli K-12. J Bacteriol. 1978 Mar;133(3):1351–1357. doi: 10.1128/jb.133.3.1351-1357.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T., Goman M., Scaife J. Lambda transducing bacteriophage carrying deletions of the argCBH-rpoBC region of the Escherichia coli chromosome. J Bacteriol. 1979 Nov;140(2):479–489. doi: 10.1128/jb.140.2.479-489.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaitis A. J., Palchaudhuri S., Glansdorff N., Maas W. K. Isolation and characterization of lambdadargECBH transducing phages and heteroduplex analysis of the argECBH cluster. Mol Gen Genet. 1976 Jan 16;143(2):185–196. doi: 10.1007/BF00266921. [DOI] [PubMed] [Google Scholar]
- Nosikov V. V., Braga E. A., Karlishev A. V., Zhuze A. L., Polyanovsky O. L. Protection of particular cleavage sites of restriction endonucleases by distamycin A and actinomycin D. Nucleic Acids Res. 1976 Sep;3(9):2293–2301. doi: 10.1093/nar/3.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E., Lee H. J., Deonier R. C., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. VI. Mapping of F14 sequences homologous to phi 80dmetBJF and phi 80dargECBH bacteriophages. J Mol Biol. 1974 Nov 15;89(4):599–618. doi: 10.1016/0022-2836(74)90038-2. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Kramer R. A., Davis R. W. Cloning of the yeast ribosomal DNA repeat unit in SstI and HindIII lambda vectors using genetic and physical size selections. J Mol Biol. 1978 Aug 15;123(3):371–386. doi: 10.1016/0022-2836(78)90085-2. [DOI] [PubMed] [Google Scholar]
- Press R., Glansdorff N., Miner P., De Vries J., Kadner R., Maas W. K. Isolation of transducing particles of phi-80 bacteriophage that carry different regions of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1971 Apr;68(4):795–798. doi: 10.1073/pnas.68.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., James A., Szybalski W. Discrete length classes of DNA depend on mode of dehydration. Proc Natl Acad Sci U S A. 1978 Feb;75(2):710–714. doi: 10.1073/pnas.75.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]