Abstract
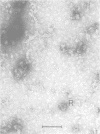
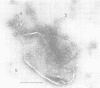
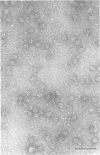
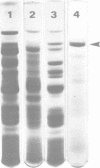
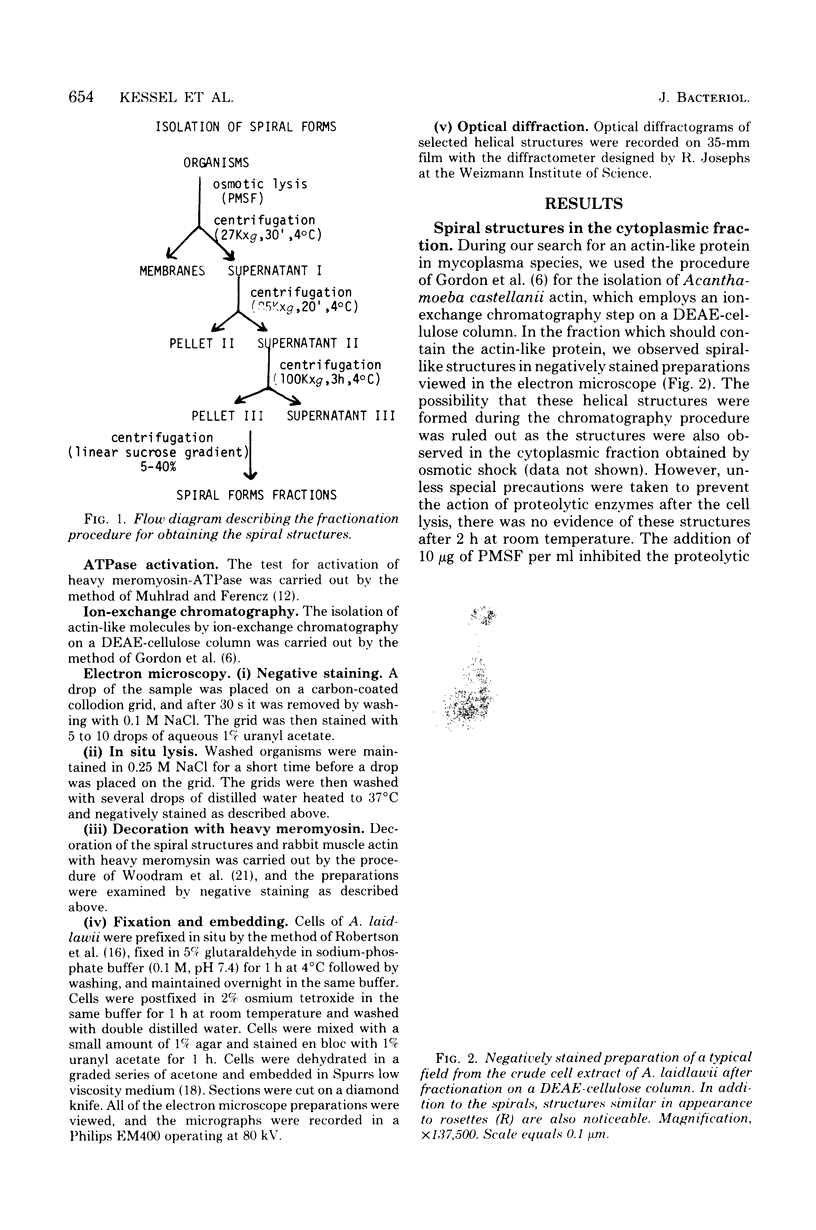
A distinct spiral protein structure was found in three species of Acholeplasma, but was not found in the Mycoplasma species studied. The spirals, which are 14 nm in width and of variable length from 50 to 300 nm, are formed by a helical arrangement of 7-nm subunits. A rosette-like structure 45 nm in diameter also composed of 7-nm subunits was found in close association with the spirals and may be a taut in vivo form of the spiral. The electrophoretic profile in sodium dodecyl sulfate-polyacrylamide gels indicated that the spirals are composed of a predominant polypeptide with an apparent molecular weight of 100,000. No evidence can be found for inferring actin-like properties for this structure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark M. W., Hammons M., Langer J. A., Lake J. A. Helical arrays of Escherichia coli small ribosomal subunits produced in vitro. J Mol Biol. 1979 Dec 5;135(2):507–512. doi: 10.1016/0022-2836(79)90450-9. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Tully J. G., Popkin T. J., Bové J. M. Morphology, ultrastructure, and bacteriophage infection of the helical mycoplasma-like organism (Spiroplasma citri gen. nov., sp. nov.) cultured from "stubborn" disease of citrus. J Bacteriol. 1973 Jul;115(1):367–384. doi: 10.1128/jb.115.1.367-386.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J., Eisenberg E., Korn E. D. Characterization of cytoplasmic actin isolated from Acanthamoeba castellanii by a new method. J Biol Chem. 1976 Aug 10;251(15):4778–4786. [PubMed] [Google Scholar]
- Josephs R., Borisy G. Self-assembly of glutamic dehydrogenase into ordered superstructures: multichain tubes formed by association of single molecules. J Mol Biol. 1972 Mar 14;65(1):127–155. doi: 10.1016/0022-2836(72)90496-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J. Analysis of the helical ribosome structures of Mycoplasma gallisepticum. Proc Natl Acad Sci U S A. 1971 Jan;68(1):43–47. doi: 10.1073/pnas.68.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J., Chaudhuri U. Gliding mycoplasmas are inhibited by cytochalasin B and contain a polymerizable protein fraction. J Supramol Struct. 1979;12(3):299–304. doi: 10.1002/jss.400120303. [DOI] [PubMed] [Google Scholar]
- Muhlrad A., Ferencz K. Studies on the properties of chemically modified actin IV: activation of myosin ATPase by actin and trinitrophenylated actin. Physiol Chem Phys. 1973;5(1):13–26. [PubMed] [Google Scholar]
- Neimark H. C. Extraction of an actin-like protein from the prokaryote Mycoplasma pneumoniae. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4041–4045. doi: 10.1073/pnas.74.9.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzio M. A., Pearson A. M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1977 Jan 25;490(1):27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Robertson J., Gomersall M., Gill P. Effect of preparatory techniques on the gross morphology of Mycoplasma hominis. J Bacteriol. 1975 Nov;124(2):1019–1022. doi: 10.1128/jb.124.2.1019-1022.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Townsend R., Burgess J., Plaskitt K. A. Morphology and ultrastructure of helical and nonhelical strains of Spiroplasma citri. J Bacteriol. 1980 Jun;142(3):973–981. doi: 10.1128/jb.142.3.973-981.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voordouw G., Veeger C., Van Breemen J. F., Van Bruggen E. F. Structure of pyridine nucleotide transhydrogenase from Azotobacter vinelandii. Eur J Biochem. 1979 Aug 1;98(2):447–454. doi: 10.1111/j.1432-1033.1979.tb13205.x. [DOI] [PubMed] [Google Scholar]
- Woodrum D. T., Rich S. A., Pollard T. D. Evidence for biased bidirectional polymerization of actin filaments using heavy meromyosin prepared by an improved method. J Cell Biol. 1975 Oct;67(1):231–237. doi: 10.1083/jcb.67.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]