Abstract
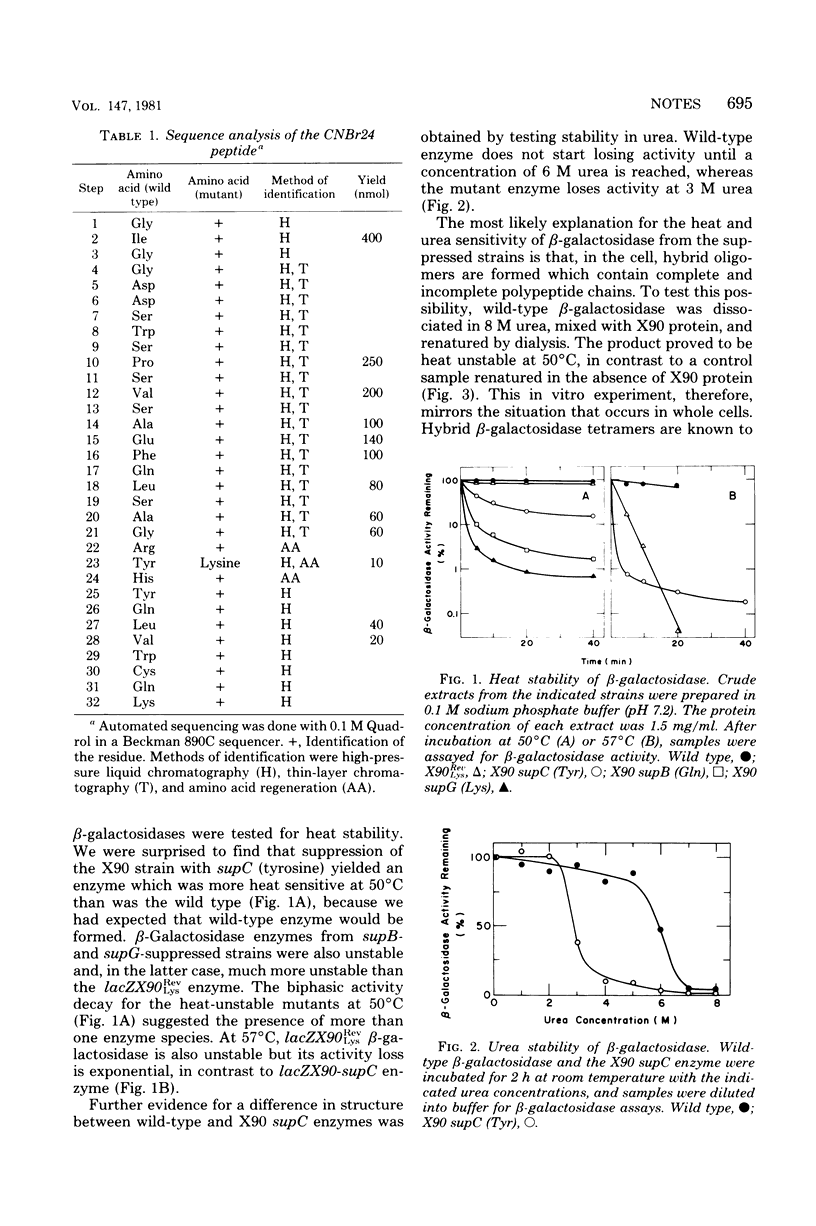
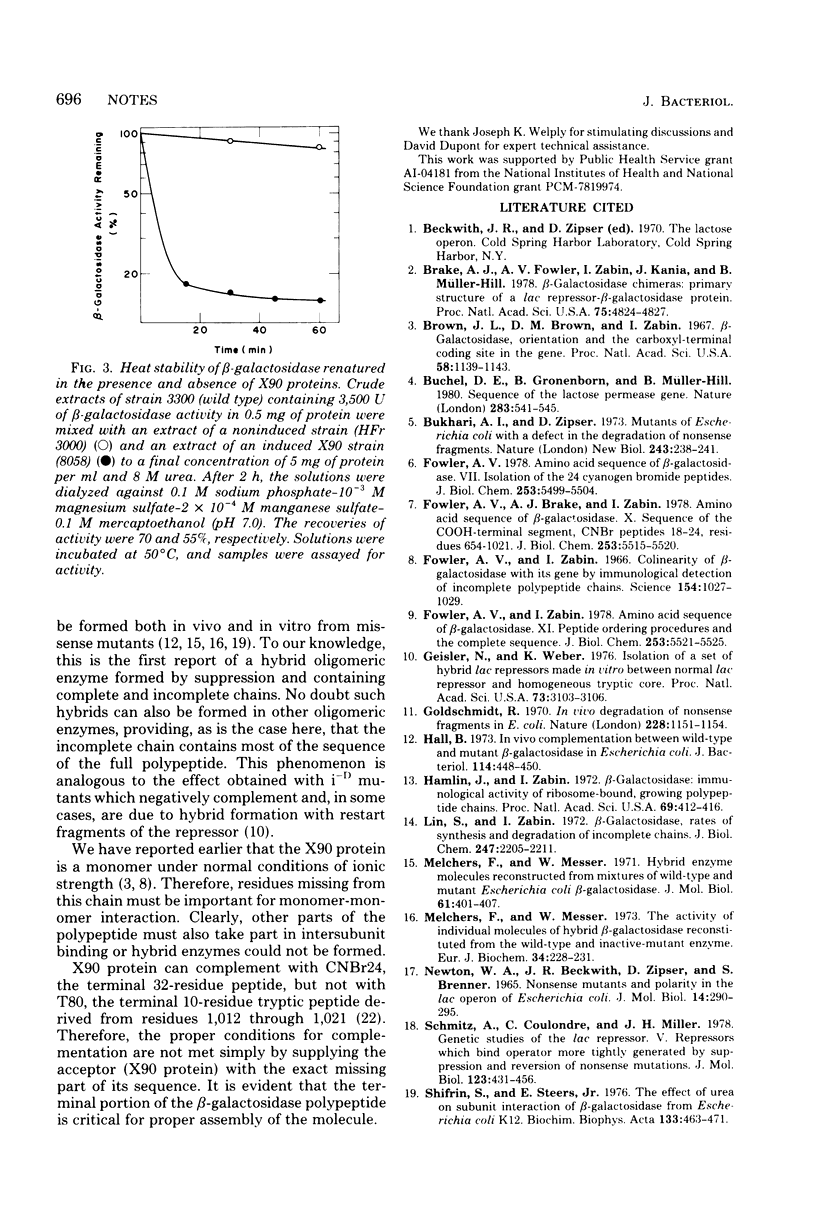
The position of the termination codon in lacZX90 was determined by isolation of a lac+ revertant. Lysine was found to replace tyrosine at position 1,012 of beta-galactosidase, indicating that X90 protein lacked the carboxyl-terminal 10 residues. A heat- and urea-sensitive hybrid enzyme was formed in vivo when supC, which supplies tyrosine to the position in the polypeptide corresponding to the nonsense codon, was used to suppress lacZX90. This result shows that suppression that adds back the original amino acid may not lead to the production of the wild-type enzyme if the latter is multimeric, because incomplete chains can be incorporated into the oligomer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brake A. J., Fowler A. V., Zabin I., Kania J., Müller-Hill B. beta-Galactosidase chimeras: primary structure of a lac repressor-beta-galactosidase protein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4824–4827. doi: 10.1073/pnas.75.10.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Brown D. M., Zabin I. Beta-galactosidase: orientation and the carboxyl-terminal coding site in the gene. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1139–1143. doi: 10.1073/pnas.58.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Fowler A. V. Amino acid sequence of beta-galactosidase. VII. Isolation of the 24 cyanogen bromide peptides. J Biol Chem. 1978 Aug 10;253(15):5499–5504. [PubMed] [Google Scholar]
- Fowler A. V., Brake A. J., Zabin I. Amino acid sequence of beta-galactosidase. X. Sequence of the COOH-terminal segment, CNBr peptides 18 to 24, residues 654 to 1021. J Biol Chem. 1978 Aug 10;253(15):5515–5520. [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Amino acid sequence of beta-galactosidase. XI. Peptide ordering procedures and the complete sequence. J Biol Chem. 1978 Aug 10;253(15):5521–5525. [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Co-linearity of beta-galactosidase with its gene by immunological detection of incomplete polypeptide chains. Science. 1966 Nov 25;154(3752):1027–1029. doi: 10.1126/science.154.3752.1027. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Isolation of a set of hybrid lac repressors made in vitro between normal lac repressor and its homogeneous tryptic core. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3103–3106. doi: 10.1073/pnas.73.9.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- Hall B. G. In vivo complementation between wild-type and mutant -galactosidase in Escherichia coli. J Bacteriol. 1973 Apr;114(1):448–450. doi: 10.1128/jb.114.1.448-450.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J., Zabin I. -Galactosidase: immunological activity of ribosome-bound, growing polypeptide chains. Proc Natl Acad Sci U S A. 1972 Feb;69(2):412–416. doi: 10.1073/pnas.69.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Zabin I. Beta-galactosidase. Rates of synthesis and degradation of incomplete chains. J Biol Chem. 1972 Apr 10;247(7):2205–2211. [PubMed] [Google Scholar]
- Melchers F., Messer W. Hybrid enzyme molecules reconstituted from mixtures of wild-type and mutant Escherichia coli -galactosidase. J Mol Biol. 1971 Oct 28;61(2):401–407. doi: 10.1016/0022-2836(71)90389-5. [DOI] [PubMed] [Google Scholar]
- Melchers F., Messer W. The activity of individual molecules of hybrid -galactosidase reconstituted from the wild-type and an inactive-mutant enzyme. Eur J Biochem. 1973 Apr;34(2):228–231. doi: 10.1111/j.1432-1033.1973.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Newton W. A., Beckwith J. R., Zipser D., Brenner S. Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol. 1965 Nov;14(1):290–296. doi: 10.1016/s0022-2836(65)80250-9. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Coulondre C., Miller J. H. Genetic studies of the lac repressor. V. Repressors which bind operator more tightly generated by suppression and reversion of nonsense mutations. J Mol Biol. 1978 Aug 15;123(3):431–454. doi: 10.1016/0022-2836(78)90089-x. [DOI] [PubMed] [Google Scholar]
- Shifrin S., Steers E., Jr The effect of urea on subunit interactions of beta-galactosidase from Escherichia coli K12. Biochim Biophys Acta. 1967 Apr 11;133(3):463–471. doi: 10.1016/0005-2795(67)90550-8. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Perrin D., Jacob F., Monod J. Identification par complémentation in vitro et purification d'un segment peptidique de la beta-galatosidase d'escherichia coli. J Mol Biol. 1965 Jul;12(3):918–923. doi: 10.1016/s0022-2836(65)80338-2. [DOI] [PubMed] [Google Scholar]
- Welply J. K., Mandecki W., Fowler A. V., Zabin I. beta-galactosidase omega-complementation with a small cyanogen bromide peptide. Biochem Biophys Res Commun. 1980 Mar 13;93(1):223–227. doi: 10.1016/s0006-291x(80)80269-5. [DOI] [PubMed] [Google Scholar]



