Abstract
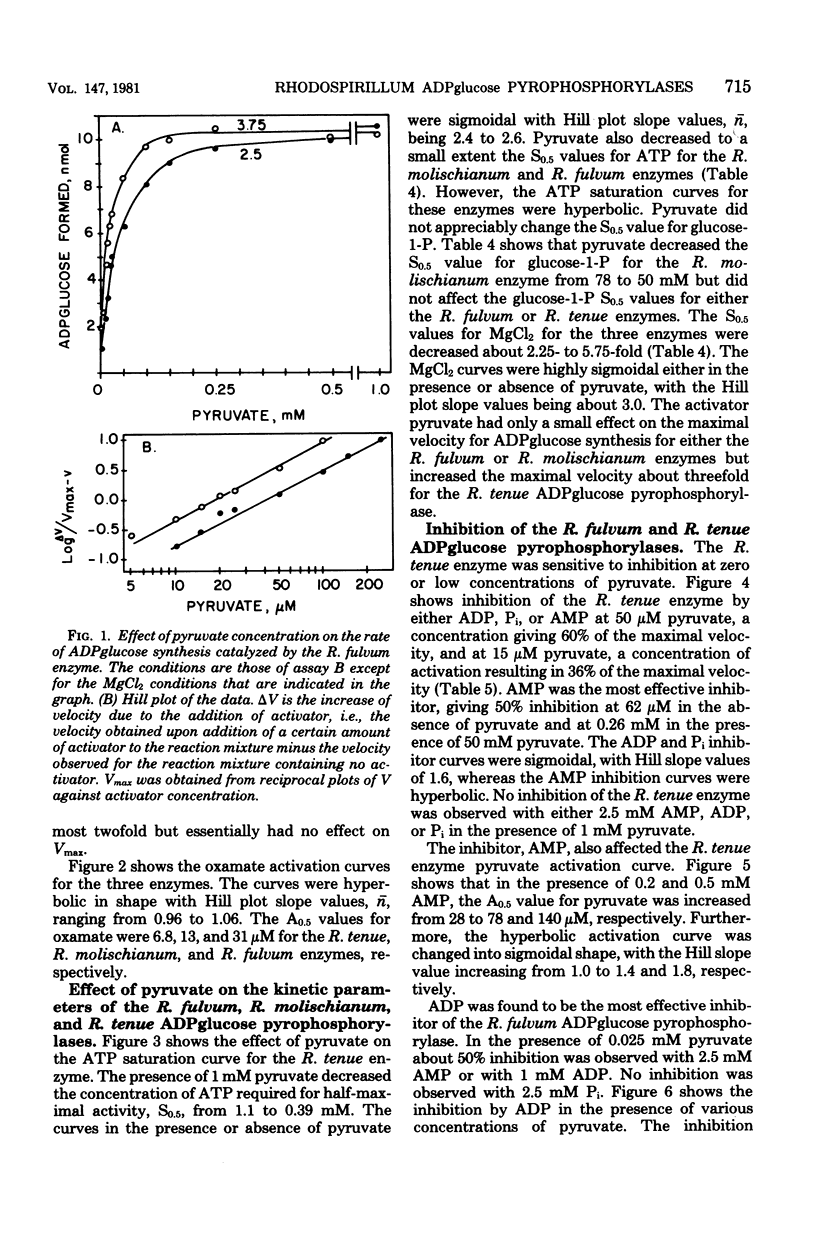
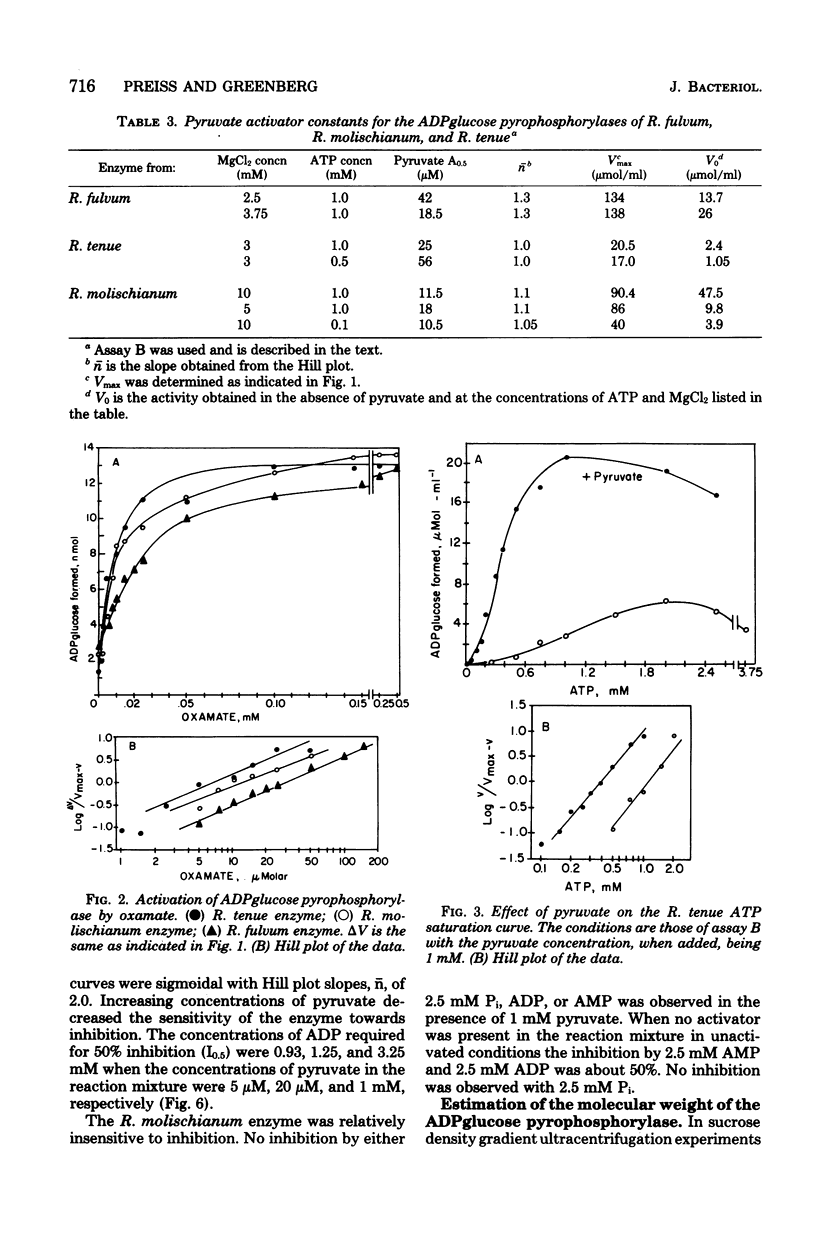
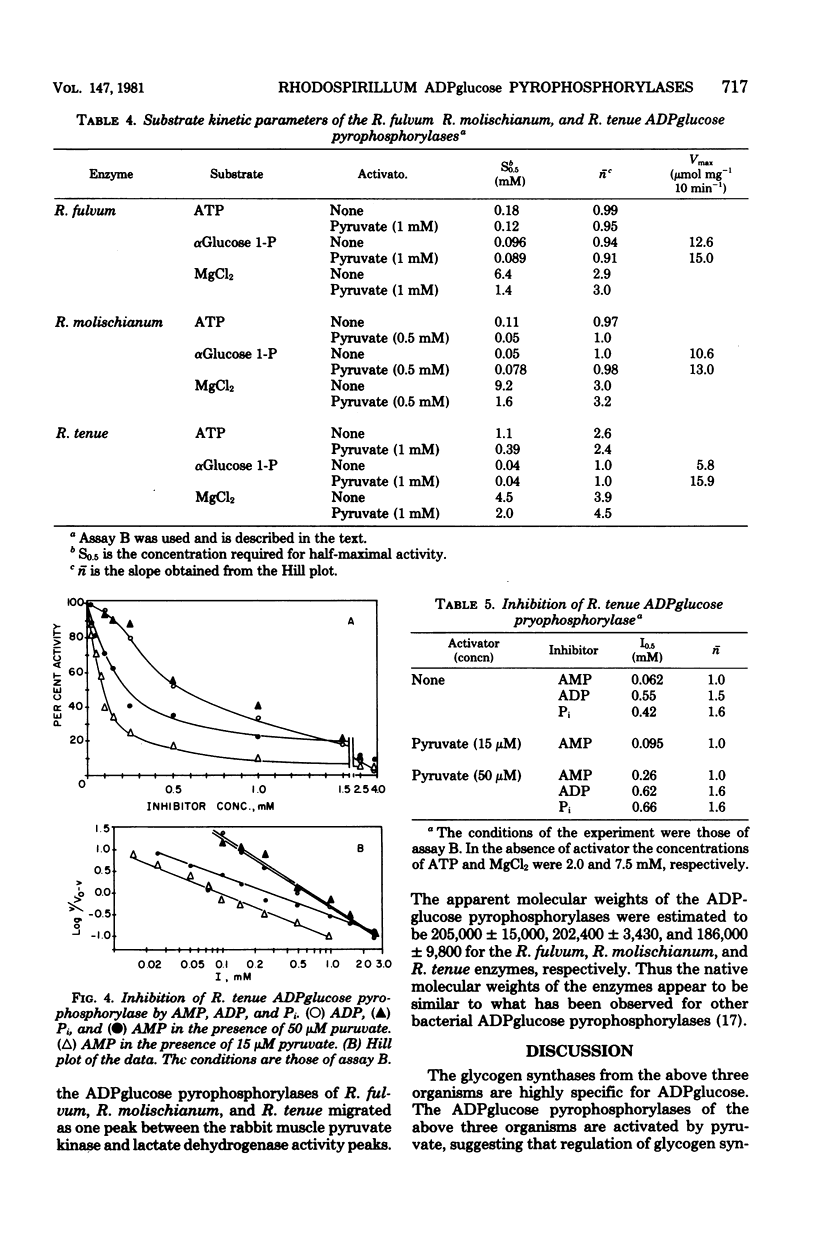
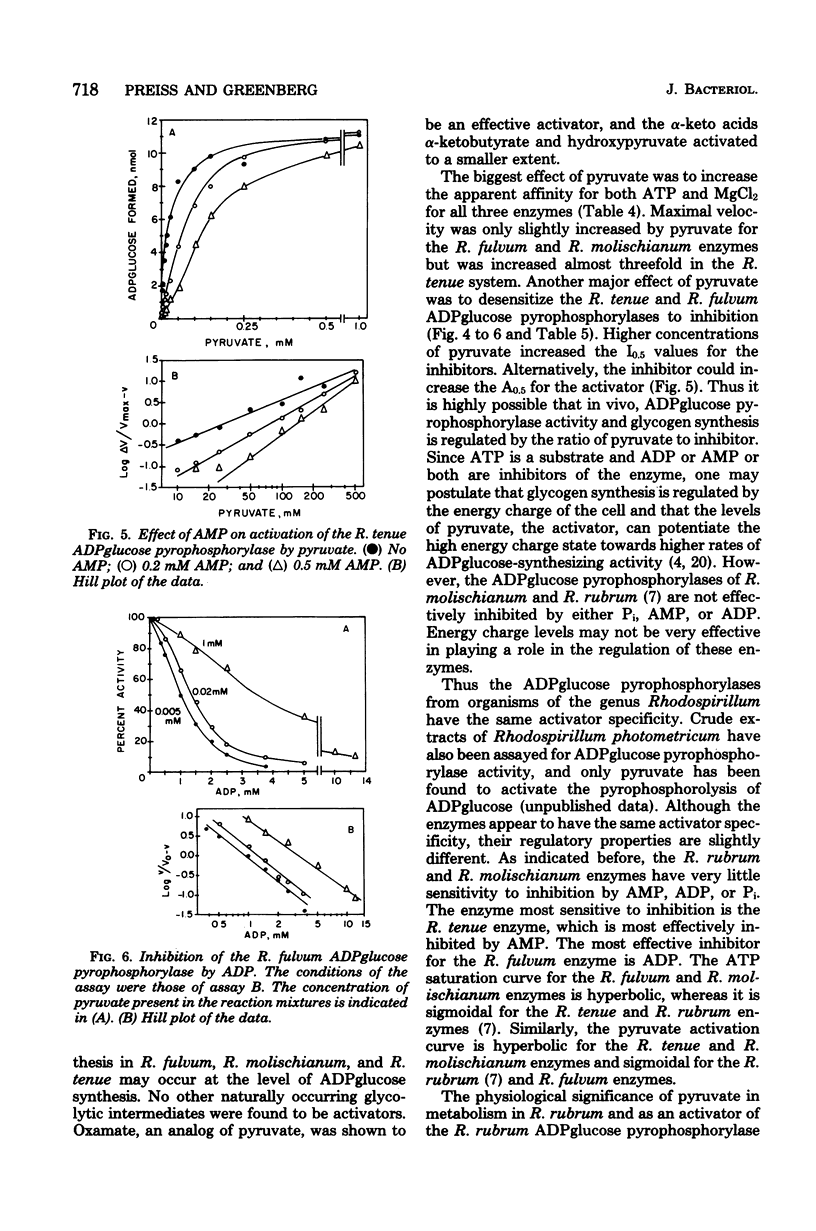
The adenosine diphosphate (ADP) glucose pyrophosphorylases from Rhodospirillum fulvum, Rhodospirillum molischianum, and Rhodospirillum tenue were partially purified, and their kinetic properties were studied. The enzyme from the three organisms was found to be activated by pyruvate and thus was similar to the Rhodospirillum rubrum enzyme that had been previously studied (C. E. Furlong, and J. Preiss, J. Biol. Chem. 244:2539-2548, 1979). The enzymes from R. fulvum, R. molischianum, and R. tenue were also activated by oxamate, an analog of pyruvate. Other alpha-keto acids, alpha-ketobutyrate and hydroxypyruvate, activated to a smaller extent. The presence of pyruvate increased the apparent affinity for adenosine 5'-triphosphate and MgCl2 for all three enzymes. The R. molischianum enzyme has very little sensitivity to inhibition by adenosine 5'-monophosphate, ADP, or inorganic phosphate. However, R. tenue ADPglucose pyrophosphorylase is very sensitive to inhibition by adenosine 5'-monophosphate, and the R. fulvum enzyme is inhibited by ADP. Increasing pyruvate concentration reversed the inhibition caused by adenosine 5'-monophosphate or ADP. Since ADPglucose is the glycosyl donor for synthesis of glycogen, it is possible that in vivo glycogen synthesis is regulated by the concentration of pyruvate and, in the case of R. fulvum and R. tenue, by the ratio of pyruvate concentration to inhibitor concentration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer C., Preiss J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli b alpha-1,4,-glucan: alpha-1,4-glucan 6-glycosyltansferase. Biochemistry. 1977 Aug 9;16(16):3693–3699. doi: 10.1021/bi00635a029. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzler D. N., Leckie M. P., Lais C. J., Magnani J. L. Evidence for the allosteric regulation of glycogen synthesis in the intact Escherichia coli cell. Agreement of the values of the parameters of the Hill equation fitted to data for glycogen synthesis in vivo with the abailable values obtained in vitro with adenosine diphosphoglucose synthetase. J Biol Chem. 1975 Mar 25;250(6):2383–2387. [PubMed] [Google Scholar]
- Fox J., Kawaguchi K., Greenberg E., Preiss J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli B ADPglucose:1,4-alpha-D-glucan 4-alpha-glucosyltransferase. Biochemistry. 1976 Feb 24;15(4):849–857. doi: 10.1021/bi00649a019. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Preiss J. Biosynthesis of bacterial glycogen. VII. Purification and properties of the adenosine diphosphoglucose pyrophosphorylase of Rhodospirillium rubrum. J Biol Chem. 1969 May 25;244(10):2539–2548. [PubMed] [Google Scholar]
- GREENBERG E., PREISS J. THE OCCURRENCE OF ADENOSINE DIPHOSPHATE GLUCOSE: GLYCOGEN TRANSGLUCOSYLASE IN BACTERIA. J Biol Chem. 1964 Dec;239:4314–4315. [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Hawker J. S., Ozbun J. L., Ozaki H., Greenberg E., Preiss J. Interaction of spinach leaf adenosine diphosphate glucose alpha-1,4-glucan alpha-4-glucosyl transferase and alpha-1,4-glucan, alpha-1,4-glucan-6-glycosyl transferase in synthesis of branched alpha-glucan. Arch Biochem Biophys. 1974 Feb;160(2):530–551. doi: 10.1016/0003-9861(74)90430-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Preiss J., Greenberg E. Biosynthesis of bacterial glycogen. 3. The adenosine diphosphate-glucose: alpha-4-glucosyl transferase of Escherichia coli B. Biochemistry. 1965 Nov;4(11):2328–2334. doi: 10.1021/bi00887a010. [DOI] [PubMed] [Google Scholar]
- Preiss J. Regulation of adenosine diphosphate glucose pyrophosphorylase. Adv Enzymol Relat Areas Mol Biol. 1978;46:317–381. doi: 10.1002/9780470122914.ch5. [DOI] [PubMed] [Google Scholar]
- Shen L. C., Atkinson D. E. Regulation of adenosine diphosphate glucose synthase from Escherichia coli. Interactions of adenylate energy charge and modifier concentrations. J Biol Chem. 1970 Aug 10;245(15):3996–4000. [PubMed] [Google Scholar]
- Sigal N., Cattanéo J., Chambost J. P., Favard A. Characterization and partial purification of a branching enzyme from Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 8;20(5):616–620. doi: 10.1016/0006-291x(65)90444-4. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Doudoroff M., Kunisawa R., Contopoulou R. THE ROLE OF ORGANIC SUBSTRATES IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1246–1260. doi: 10.1073/pnas.45.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]


