Abstract
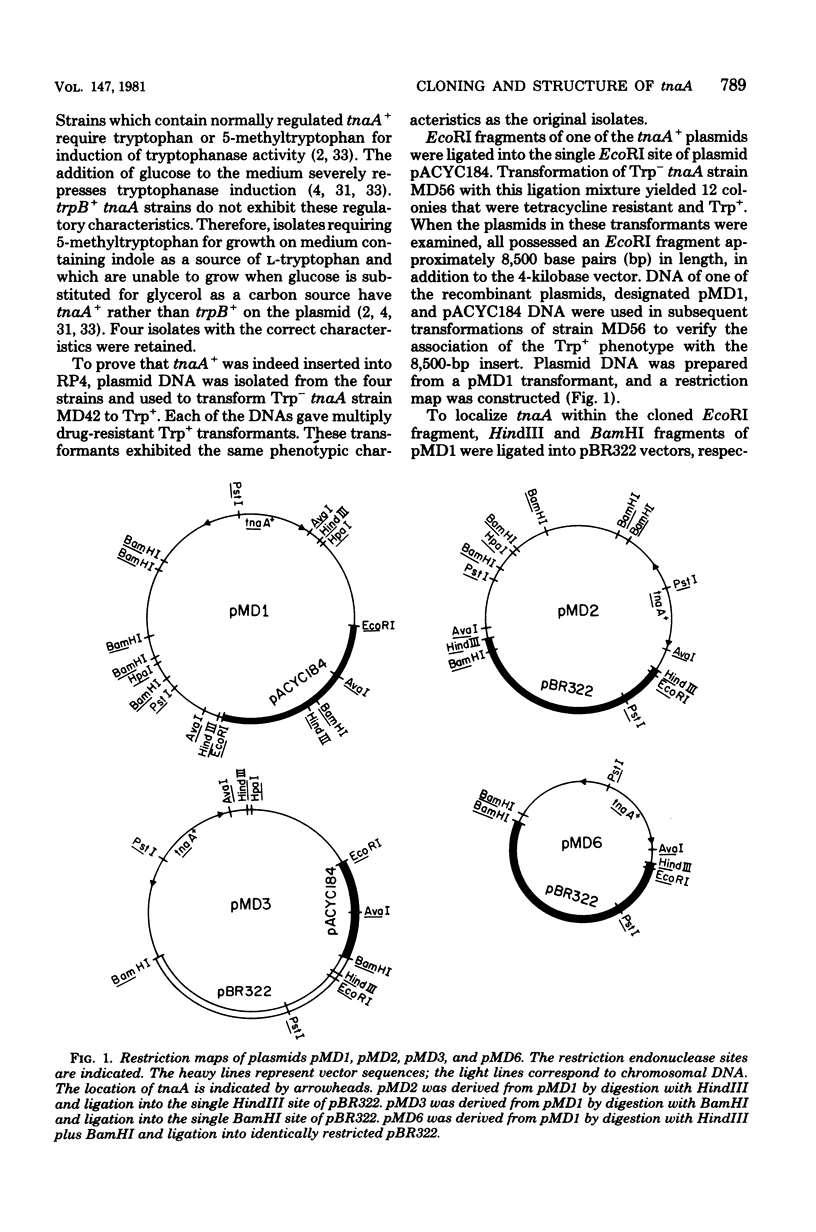
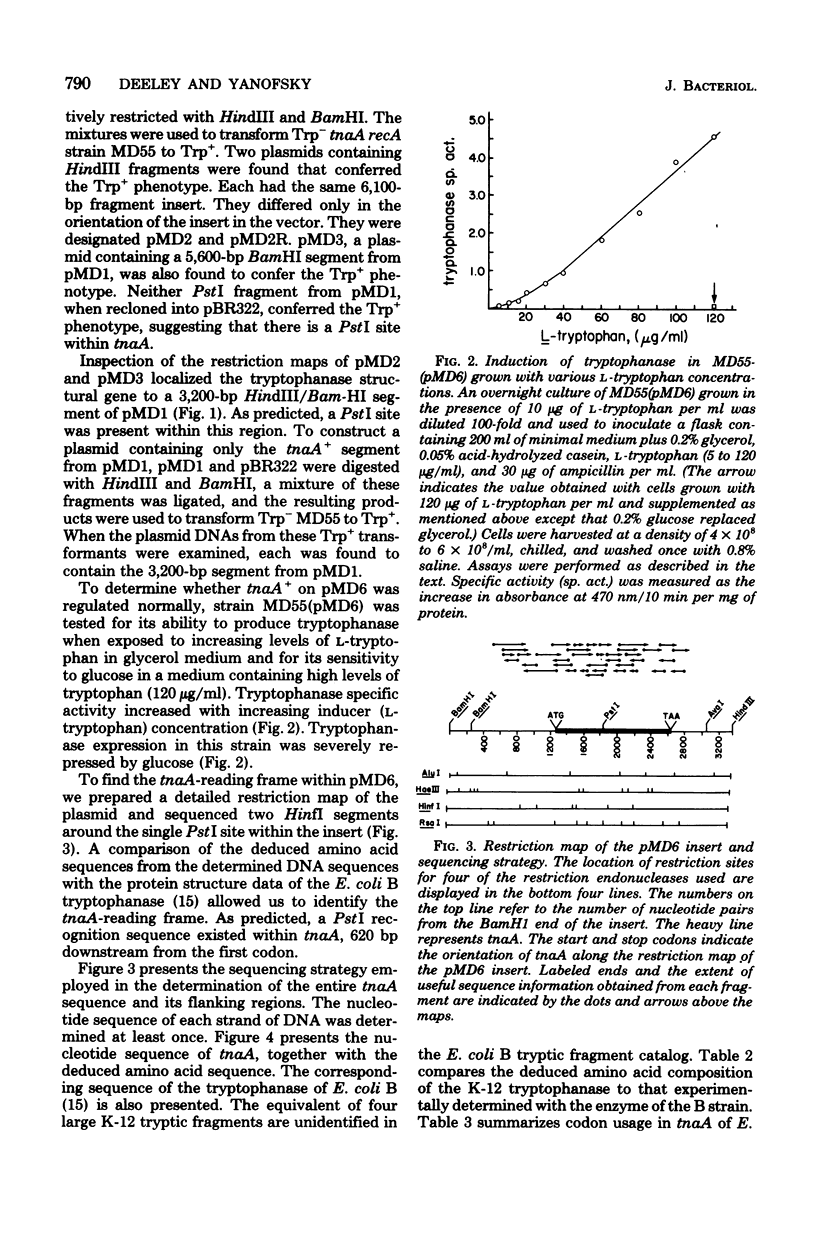
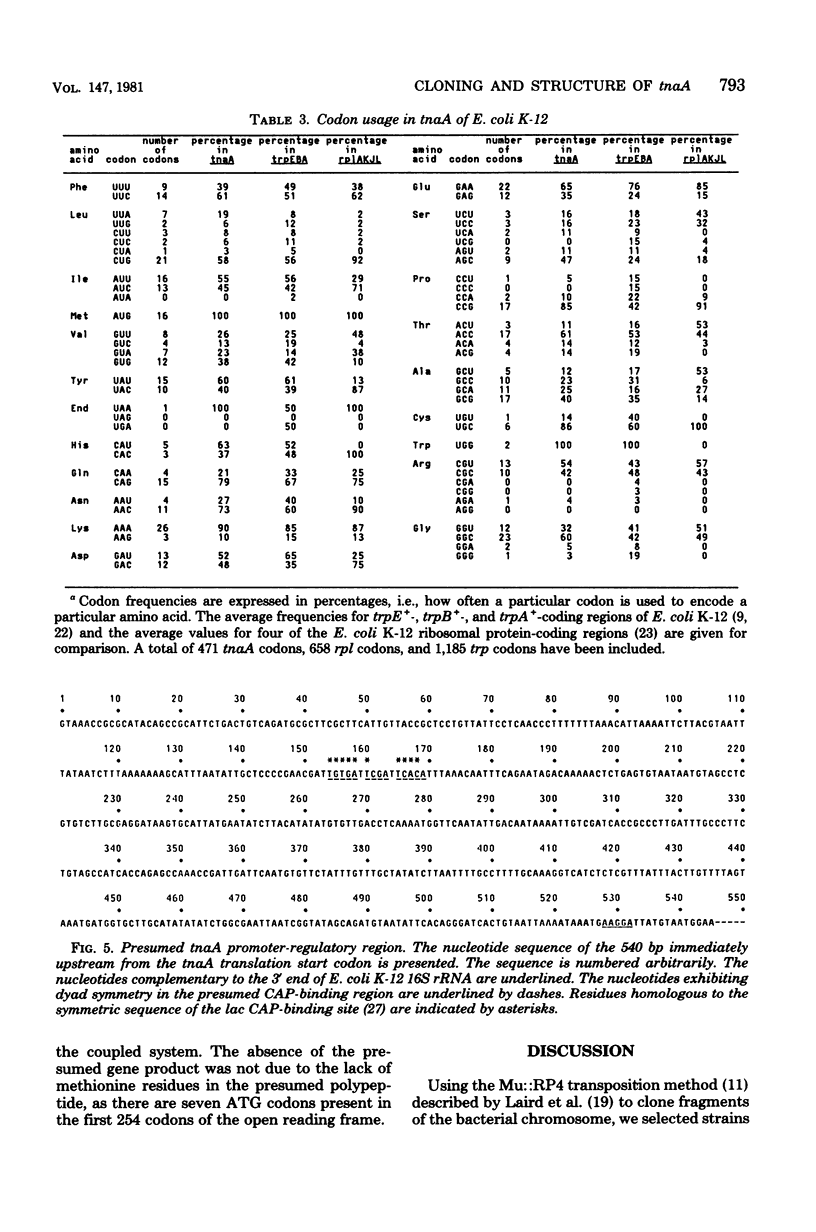
The tryptophanase structural gene, tnaA, of Escherichia coli K-12 was cloned and sequenced. The size, amino acid composition, and sequence of the protein predicted from the nucleotide sequence agree with protein structure data previously acquired by others for the tryptophanase of E. coli B. Physiological data indicated that the region controlling expression of tnaA was present in the cloned segment. Sequence data suggested that a second structural gene of unknown function was located distal to tnaA and may be in the same operon. The pattern of codon usage in tnaA was intermediate between codon usage in four of the ribosomal protein structural genes and the structural genes for three of the tryptophan biosynthetic proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS R. O., DEMOSS R. D. Properties of tryptophanase from Escherichia coli. Biochim Biophys Acta. 1962 Dec 4;65:233–244. doi: 10.1016/0006-3002(62)91042-9. [DOI] [PubMed] [Google Scholar]
- BURROUS S. E., DEMOSS R. D. STUDIES ON TRYPTOPHAN PERMEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 6;73:623–637. doi: 10.1016/0006-3002(63)90332-9. [DOI] [PubMed] [Google Scholar]
- Bennett G. N., Yanofsky C. Sequence analysis of operator constitutive mutants of the tryptophan operon of Escherichia coli. J Mol Biol. 1978 May 15;121(2):179–192. doi: 10.1016/s0022-2836(78)80004-7. [DOI] [PubMed] [Google Scholar]
- Bilezikian J. P., Kaempfer R. O., Magasanik B. Mechanism of tryptophanase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):495–506. doi: 10.1016/0022-2836(67)90054-x. [DOI] [PubMed] [Google Scholar]
- Botsford J. L., DeMoss R. D. Catabolite repression of tryptophanase in Escherichia coli. J Bacteriol. 1971 Jan;105(1):303–312. doi: 10.1128/jb.105.1.303-312.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Nichols B. P., Yanofsky C. Nucleotide sequence of the trpB gene in Escherichia coli and Salmonella typhimurium. J Mol Biol. 1980 Oct 5;142(4):489–502. doi: 10.1016/0022-2836(80)90259-4. [DOI] [PubMed] [Google Scholar]
- DeMoss R. D., Moser K. Tryptophanase in diverse bacterial species. J Bacteriol. 1969 Apr;98(1):167–171. doi: 10.1128/jb.98.1.167-171.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Zurawski G., Yanofsky C. Structural and functional analysis of cloned deoxyribonucleic acid containing the trpR-thr region of the Escherichia coli chromosome. J Bacteriol. 1979 Oct;140(1):106–113. doi: 10.1128/jb.140.1.106-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Kagamiyama H., Matsubara H., Snell E. E. The chemical structure of tryptophanase from Escherichia coli. 3. Isolation and amino acid sequence of the tryptic peptides. J Biol Chem. 1972 Mar 10;247(5):1576–1586. [PubMed] [Google Scholar]
- Kagamiyama H., Wada H., Matsubara H., Snell E. E. The chemical structure of tryptophanase from Escherichia coli. II. The structure of tryptophanase monomer. J Biol Chem. 1972 Mar 10;247(5):1571–1575. [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Queen C. L., Wegman M. N. Computer analysis of nucleic acid regulatory sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4401–4405. doi: 10.1073/pnas.74.10.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A. J., Ribbons D. W., Woodrow G. C., Young I. G. Bacteriophage Mu-mediated gene transposition and in vitro cloning of the enterochelin gene cluster of Escherichia coli. Gene. 1980 Nov;11(3-4):347–357. doi: 10.1016/0378-1119(80)90074-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. CATALYTIC PROPERTIES OF TRYPTOPHANASE, A MULTIFUNCTIONAL PYRIDOXAL PHOSPHATE ENZYME. Proc Natl Acad Sci U S A. 1964 Mar;51:382–389. doi: 10.1073/pnas.51.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., van Cleemput M., Yanofsky C. Nucleotide sequence of Escherichia coli trpE. Anthranilate synthetase component I contains no tryptophan residues. J Mol Biol. 1981 Feb 15;146(1):45–54. doi: 10.1016/0022-2836(81)90365-x. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Selker E., Brown K., Yanofsky C. Mitomycin C-induced expression of trpA of Salmonella typhimurium inserted into the plasmid ColE1. J Bacteriol. 1977 Jan;129(1):388–394. doi: 10.1128/jb.129.1.388-394.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. B. Interaction of the cAMP receptor protein with the lac promoter. Nucleic Acids Res. 1980 Feb 25;8(4):759–766. [PMC free article] [PubMed] [Google Scholar]
- Snell E. E. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol. 1975;42:287–333. doi: 10.1002/9780470122877.ch6. [DOI] [PubMed] [Google Scholar]
- Suelter C. H., Wang J., Snell E. E. Direct spectrophotometric assay of tryptophanase. FEBS Lett. 1976 Jul 15;66(2):230–232. doi: 10.1016/0014-5793(76)80510-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Ward D. F., Yudkin M. D. Mutations in Escherichia coli that relieve catabolite repression of tryptophanase synthesis. Tryptophanase promoter-like mutations. J Gen Microbiol. 1976 Jan;92(1):133–137. doi: 10.1099/00221287-92-1-133. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Snell E. E. Reversibility of the tryptophanase reaction: synthesis of tryptophan from indole, pyruvate, and ammonia. Proc Natl Acad Sci U S A. 1972 May;69(5):1086–1090. doi: 10.1073/pnas.69.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin M. D. Mutations in Escherichia coli that relieve catabolite repression of tryptophanase synthesis. Mutations distant from the tryptophanase gene. J Gen Microbiol. 1976 Jan;92(1):125–132. doi: 10.1099/00221287-92-1-125. [DOI] [PubMed] [Google Scholar]
- Zubay G., Morse D. E., Schrenk W. J., Miller J. H. Detection and isolation of the repressor protein for the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1100–1103. doi: 10.1073/pnas.69.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]




