Abstract
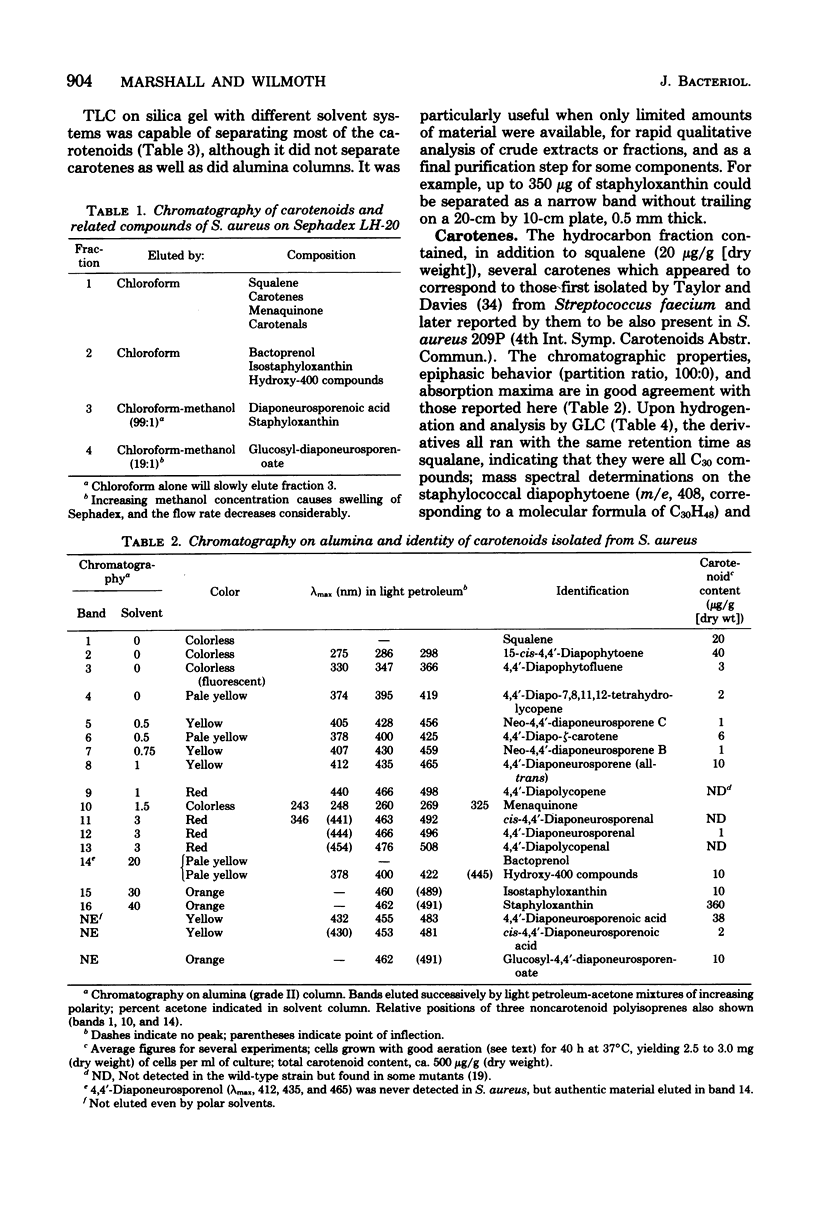
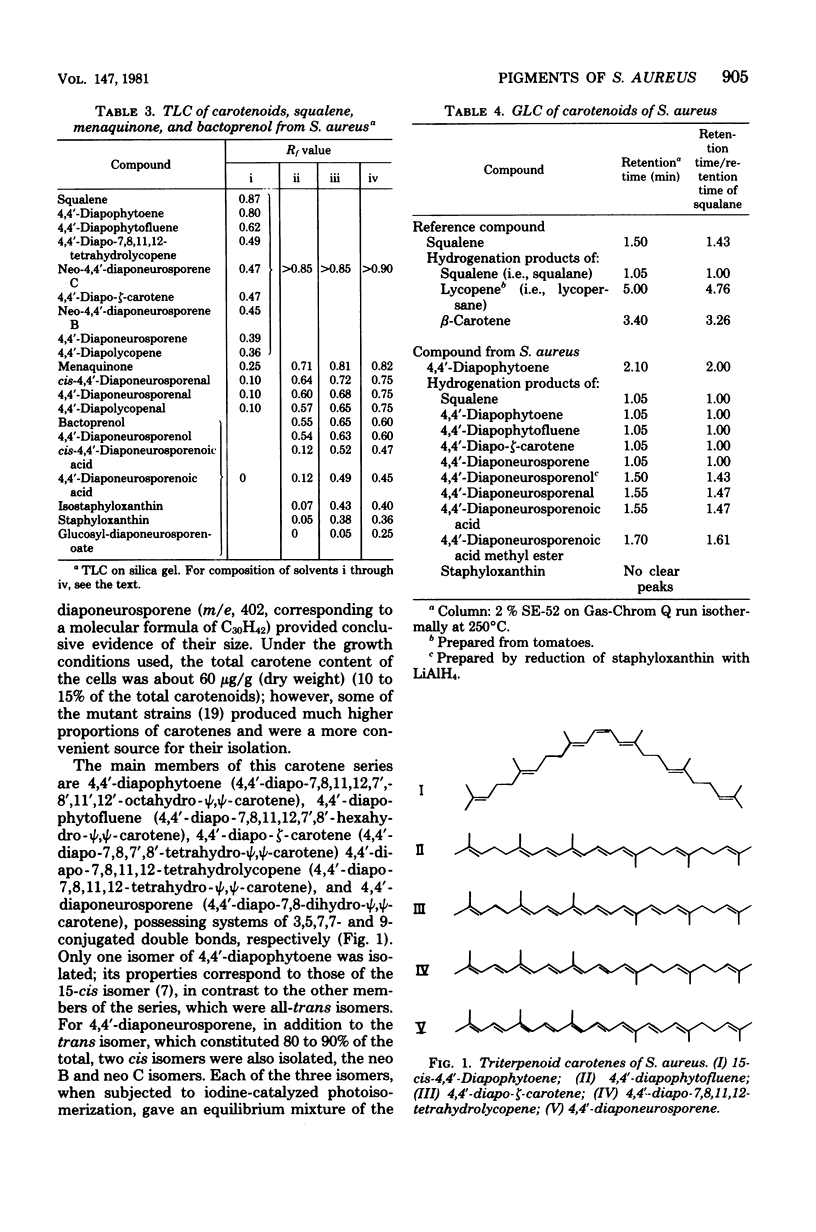
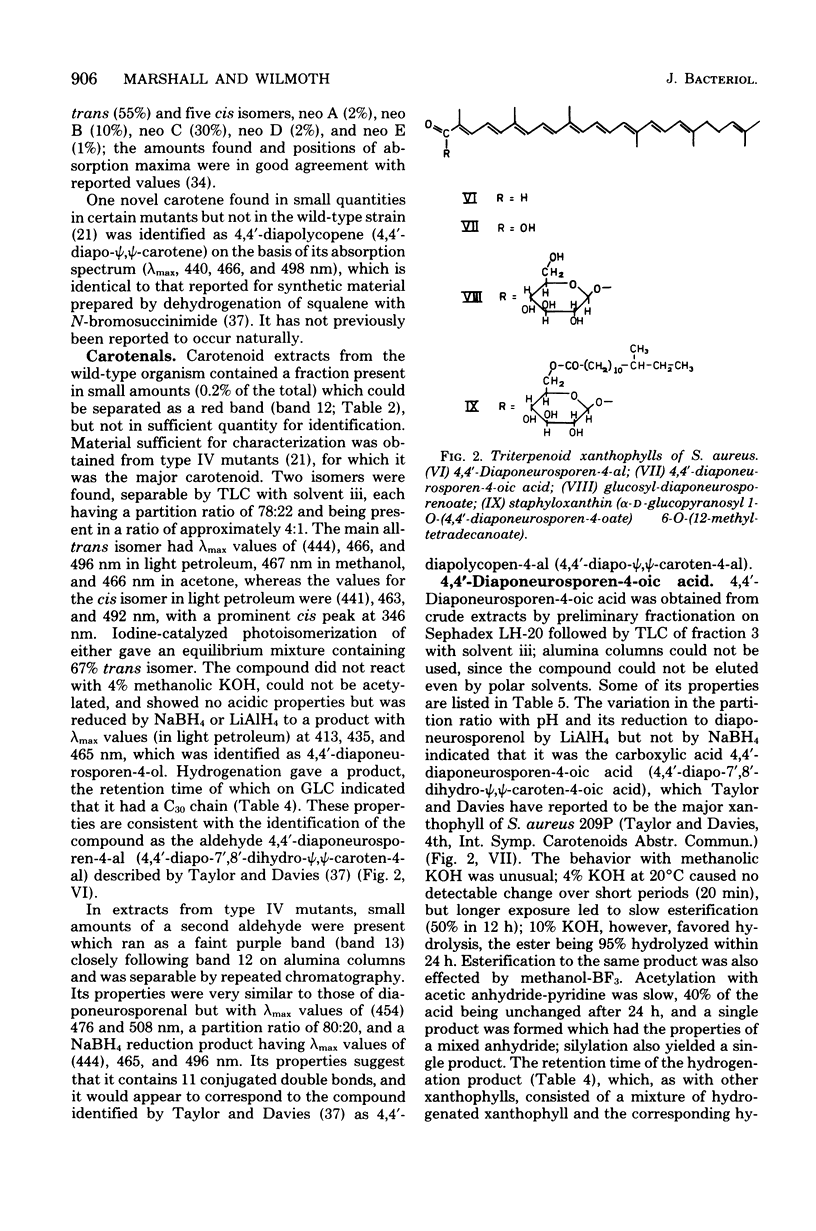
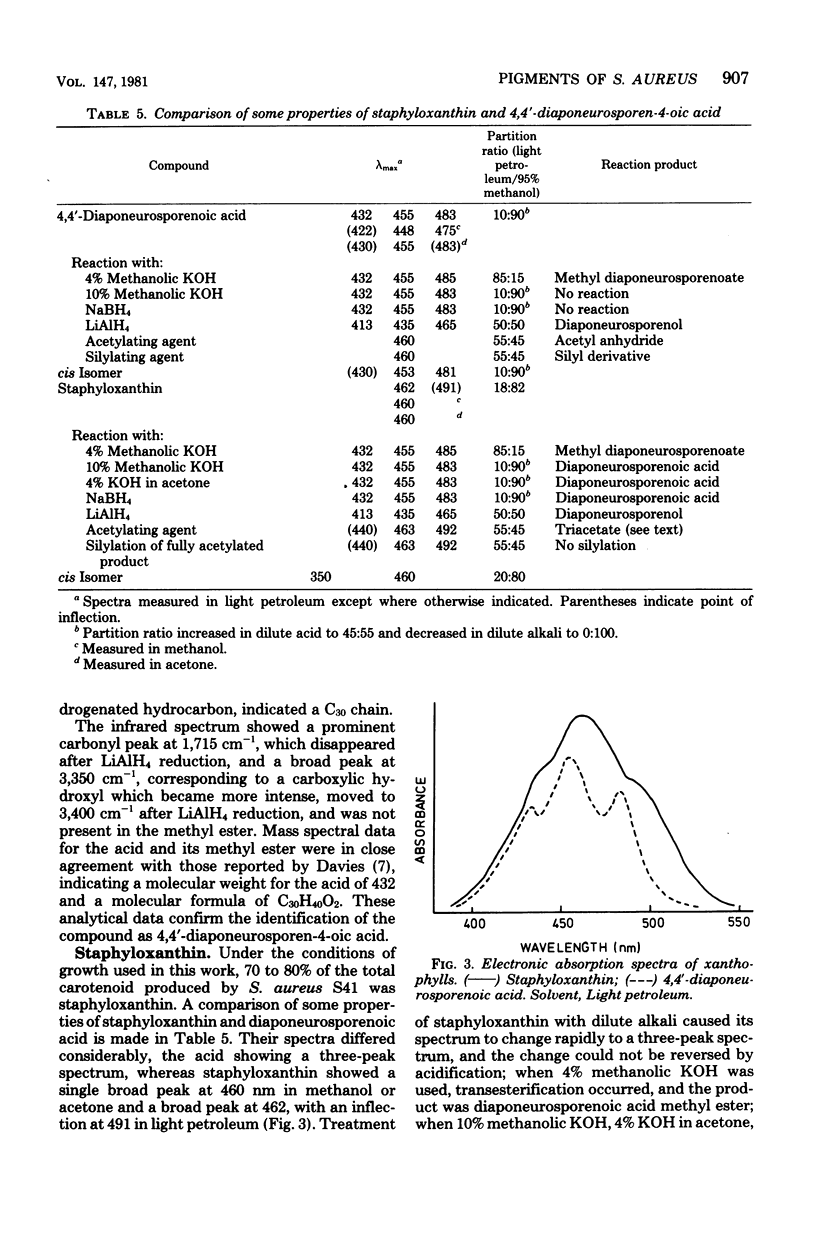
The pigments of Staphylococcus aureus were isolated and purified, and their chemical structures were determined. All of the 17 compounds identified were triterpenoid carotenoids possessing a C30 chain instead of the C40 carotenoid structure found in most other organisms. The main pigment, staphyloxanthin, was shown to be alpha-D-glucopyranosyl 1-O-(4,4'-diaponeurosporen-4-oate) 6-O-(12-methyltetradecanoate), in which glucose is esterified with both a triterpenoid carotenoid carboxylic acid and a C15 fatty acid. It is accompanied by isomers containing other hexoses and homologs containing C17 fatty acids. The carotenes 4,4'-diapophytoene, 4,4'-diapophytofluene, 4-4'-diapophytofluene, 4-4'-diapo-zeta-carotene, 4,4'-diapo-7,8,11,12-tetrahydrolycopene, and 4,4'-diaponeurosporene and the xanthophylls 4,4'-diaponeurosporenal, 4,4'-diaponeurosporenoic acid, and glucosyl diaponeurosporenoate were also identified, together with some of their isomers or breakdown products. The symmetrical 4,4'-diapo- structure was adopted for these triterpenoid carotenoids, but an alternative unsymmetrical 8'-apo-structure could not be excluded.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEGRA G., NIUTTA R., GIUFFRIDA G. Studi sulla carotenogenesi e sul ruolo dei carotenoidi nello Staphylococcus aureus. I. Azione del glucosio sulla cromogenesi dello Staphylococcus aureus (stipite Oxford). Riv Ist Sieroter Ital. 1954 May-Jun;29(3):263–269. [PubMed] [Google Scholar]
- Aasen A. J., Jensen S. L. Carotenoids of flexibacteria. 3. The structures of flexixanthin and deoxy-flexixanthin. Acta Chem Scand. 1966;20(7):1970–1988. doi: 10.3891/acta.chem.scand.20-1970. [DOI] [PubMed] [Google Scholar]
- Douglas R. J., Parisi J. T. Chromogenesis by variants of Staphylococcus aureus. J Bacteriol. 1966 Nov;92(5):1578–1579. doi: 10.1128/jb.92.5.1578-1579.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Carotenoid formation by Staphylococcus aureus. J Bacteriol. 1970 Jul;103(1):191–198. doi: 10.1128/jb.103.1.191-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinig H., Reichenbach H., Achenbach H. Carotenoid pigments of Stigmatella aurantiaca (Myxobacterales). II. Acylated carotenoid glucosides. Arch Mikrobiol. 1970;74(3):223–234. doi: 10.1007/BF00408883. [DOI] [PubMed] [Google Scholar]
- Kleinig H., Reichenbach H. Carotenoid glucosides and menaquinones from the gliding bacterium Herpetosiphon giganteus Hp a2. Arch Microbiol. 1977 Apr 1;112(3):307–310. doi: 10.1007/BF00413098. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Pugh E. L., Kramer J. K., Kates M. Isolation and identification of dehydrosqualene and C 40 -carotenoid pigments in Halobacterium cutirubrum. Biochim Biophys Acta. 1972 Mar 23;260(3):492–506. doi: 10.1016/0005-2760(72)90064-1. [DOI] [PubMed] [Google Scholar]
- Marshall J. H., Wilmoth G. J. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: evidence from a study of mutants. J Bacteriol. 1981 Sep;147(3):914–919. doi: 10.1128/jb.147.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER J. W., ANDERSON D. G. The biosynthesis of carotenes. Arch Biochem Biophys. 1962 Jun;97:520–528. doi: 10.1016/0003-9861(62)90116-9. [DOI] [PubMed] [Google Scholar]
- PORTER J. W., LINCOLN R. E. Lycopersicon selections containing a high content of carotenes and colorless polyenes; the mechanism of carotene biosynthesis. Arch Biochem. 1950 Jul;27(2):390–403. [PubMed] [Google Scholar]
- Pfander H., Wittwer F. Carotinoid-Glykoside. Untersuchungen zur Carotinoid-Zeusammensetzung im Safran. Helv Chim Acta. 1975 Sep 24;58(6):1608–1620. doi: 10.1002/hlca.19750580615. [DOI] [PubMed] [Google Scholar]
- STEUER W. Untersuchungen über die Pigmentbildung von Micrococcus pyogenes. Zentralbl Bakteriol Orig. 1956 Nov;167(3):210–216. [PubMed] [Google Scholar]
- Schindler J. Pigment production of Staphylococcus aureus PS 80 and its mutants. Zentralbl Bakteriol Orig A. 1972 Jul;221(2):157–165. [PubMed] [Google Scholar]
- Schleifer K. H., Kocur M. Classification of staphylococci based on chemical and biochemical properties. Arch Mikrobiol. 1973 Oct 4;93(1):65–85. doi: 10.1007/BF00666081. [DOI] [PubMed] [Google Scholar]
- Sobin B., Stahly G. L. The Isolation and Absorption Spectrum Maxima of Bacterial Carotenoid Pigments. J Bacteriol. 1942 Sep;44(3):265–276. doi: 10.1128/jb.44.3.265-276.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue G., Tsukada K., Tanaka S. A new triterpenoid from a mutant of Staphylococcus aureus. Biochim Biophys Acta. 1967 Aug 8;144(1):186–188. [PubMed] [Google Scholar]
- Suzue G., Tsukada K., Tanaka S. Occurrence of dehydrosqualene (C30 phytoene) in Staphylococcus aureus. Biochim Biophys Acta. 1968 Sep 2;164(1):88–93. doi: 10.1016/0005-2760(68)90074-x. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. Gas-liquid chromatography of carotenoids and other terpenoids. J Chromatogr. 1975 Jan 22;103(2):327–340. doi: 10.1016/s0021-9673(00)87224-6. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. The influence of culture conditions on carotenogenesis in Streptococcus faecium UNH564P. J Gen Microbiol. 1976 Feb;92(2):325–334. doi: 10.1099/00221287-92-2-325. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. Triterpenoid carotenoids and related lipids. The triterpenoid carotenes of Streptococcus faecium UNH 564P. Biochem J. 1974 Jun;139(3):751–760. doi: 10.1042/bj1390751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. Triterpenoid carotenoids and related lipids. Triterpenoid carotenoid aldehydes from Streptococcus faecium UNH 564P. Biochem J. 1976 Feb 1;153(2):233–239. doi: 10.1042/bj1530233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. Triterpenoid carotenoids and related lipids. Triterpenoid monohydroxy- and monoglucosyloxy-carotenoids from Streptococcus faecium UNH 564P. Biochem J. 1974 Jun;139(3):761–769. doi: 10.1042/bj1390761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDI D. DUENNSCHICHT-CHROMATOGRAPHIE EINIGER ZUCKER UND ZUCKERALKOHOLE. J Chromatogr. 1965 May;18:417–418. doi: 10.1016/s0021-9673(01)80390-3. [DOI] [PubMed] [Google Scholar]
- WILLIS A. T., JACOBS S. I., GOODBURN G. M. PIGMENT PRODUCTION, ENZYMATIC ACTIVITY AND ANTIBIOTIC SENSITIVITY OF STAPHYLOCOCCI: SUBDIVISION OF THE PATHOGENIC GROUP. J Pathol Bacteriol. 1964 Jan;87:157–167. doi: 10.1002/path.1700870122. [DOI] [PubMed] [Google Scholar]
- WILLIS A. T., TURNER G. C. Staphylococcal lipolysis and pigmentation. J Pathol Bacteriol. 1962 Oct;84:337–347. doi: 10.1002/path.1700840208. [DOI] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



