Abstract
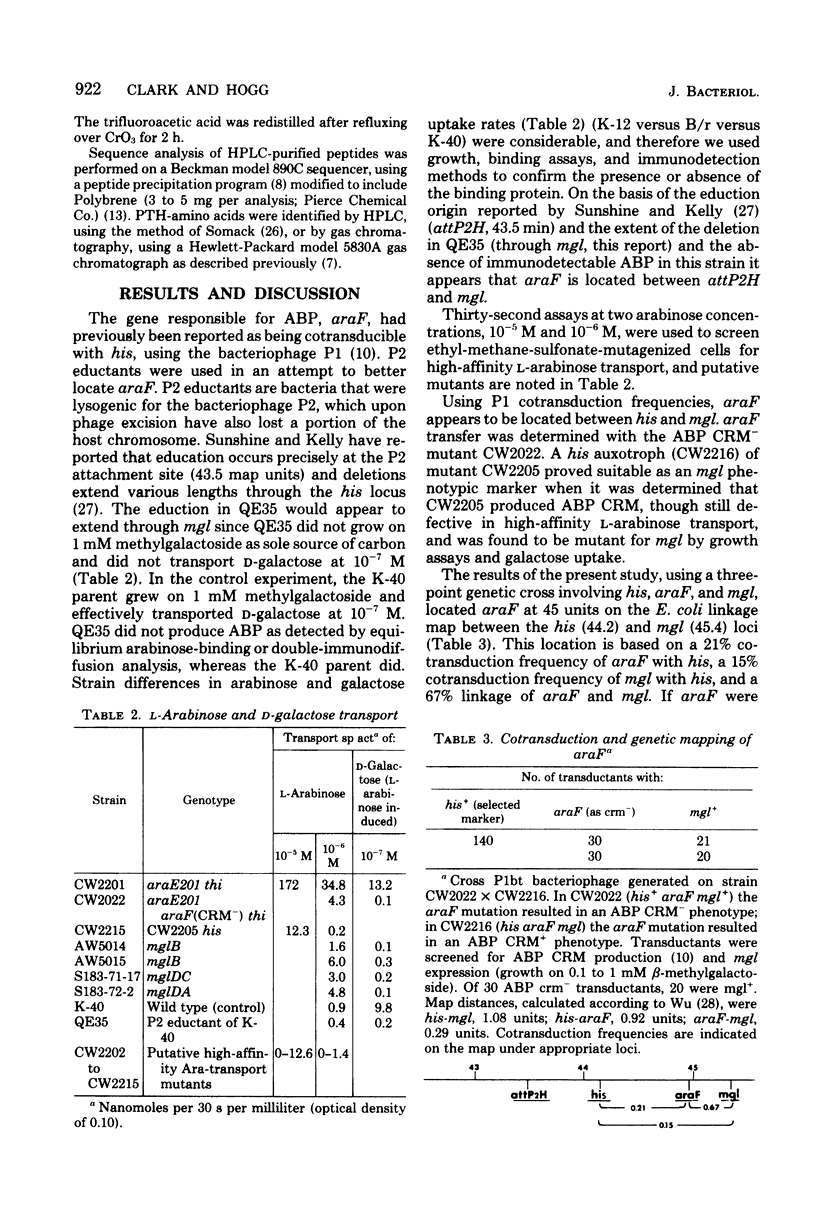
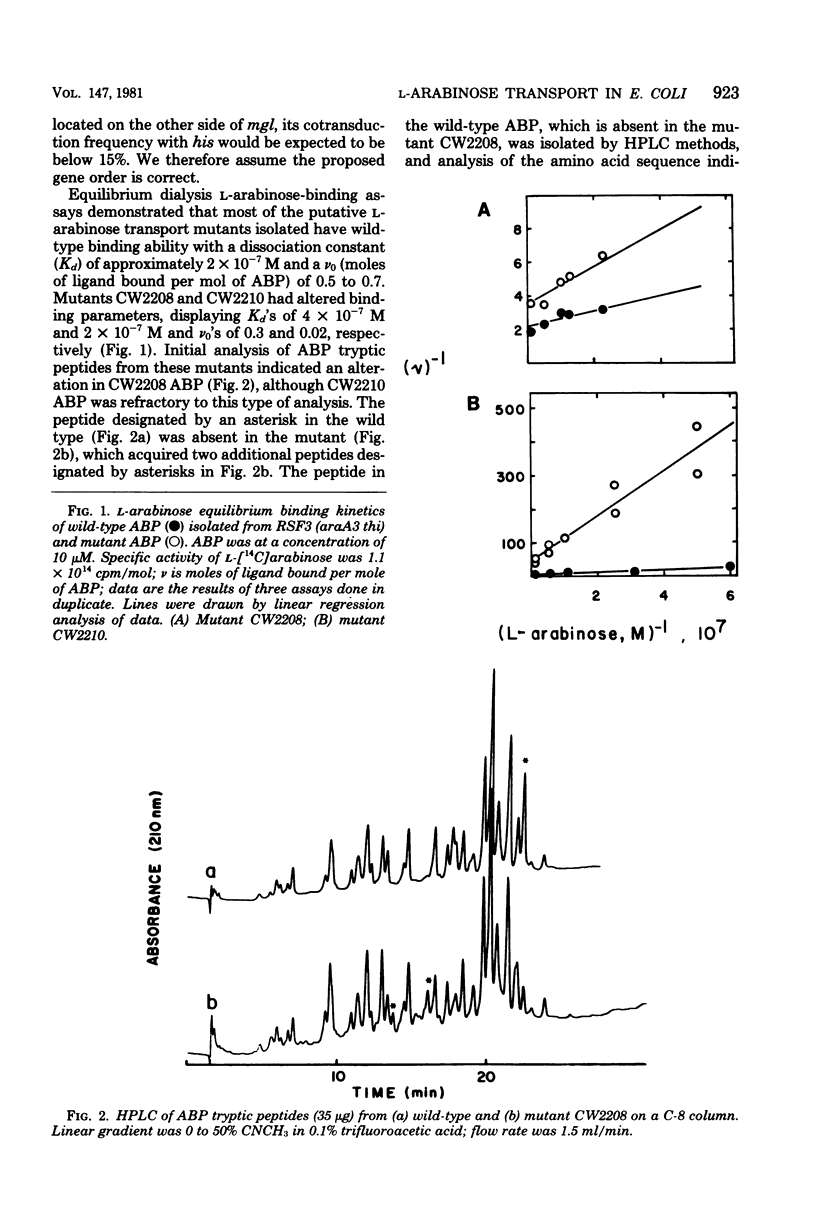
The gene araF, the product of which is the L-arabinose-binding protein--a component of the high-affinity L-arabinose transport system, was located on the Escherichia coli linkage map at 45 min. We established this location using bacteriophage P2 eductates and bacteriophage P1 cotransduction frequencies with the adjacent genetic loci, his (histidine biosynthesis) and mgl (methylgalactoside transport). In addition, we isolated a number of mutants that phenotypically exhibited altered high-affinity L-arabinose transport capacities. At least two of these mutations were located in the araF gene, as binding protein purified from these strains exhibited altered in vitro arabinose-binding properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Brown C. E., Hogg R. W. A second transport system for L-arabinose in Escherichia coli B-r controlled by the araC gene. J Bacteriol. 1972 Aug;111(2):606–613. doi: 10.1128/jb.111.2.606-613.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Doyle M. E., Brown C., Hogg R. W., Helling R. B. Induction of the ara operon of Escherichia coli B-r. J Bacteriol. 1972 Apr;110(1):56–65. doi: 10.1128/jb.110.1.56-65.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Wilcox G. Regulation: positive control. Annu Rev Genet. 1974;8:219–242. doi: 10.1146/annurev.ge.08.120174.001251. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Hermodson M., Schmer G., Kurachi K. Isolation, crystallization, and primary amino acid sequence of human platelet factor 4. J Biol Chem. 1977 Sep 25;252(18):6276–6279. [PubMed] [Google Scholar]
- Hogg R. W. "In vivo" detection of L-arabinose-binding protein, CRM-negative mutants. J Bacteriol. 1971 Feb;105(2):604–608. doi: 10.1128/jb.105.2.604-608.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. W., Englesberg E. L-arabinose binding protein from Escherichia coli B-r. J Bacteriol. 1969 Oct;100(1):423–432. doi: 10.1128/jb.100.1.423-432.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. W., Hermodson M. A. Amino acid sequence of the L-arabinose-binding protein from Escherichia coli B/r. J Biol Chem. 1977 Jul 25;252(14):5135–5141. [PubMed] [Google Scholar]
- Hogg R. W. L-Arabinose transport and the L-arabinose binding protein of Escherichia coli. J Supramol Struct. 1977;6(3):411–417. doi: 10.1002/jss.400060314. [DOI] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr A response regulator model in a simple sensory system. Science. 1977 Jun 3;196(4294):1055–1063. doi: 10.1126/science.870969. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- Newcomer M. E., Miller D. M., 3rd, Quiocho F. A. Location of the sugar-binding site of L-arabinose-binding protein. Sugar derivative syntheses, sugar binding specificity, and difference Fourier analyses. J Biol Chem. 1979 Aug 25;254(16):7529–7533. [PubMed] [Google Scholar]
- Novotny C. P., Englesberg E. The L-arabinose permease system in Escherichia coli B/r. Biochim Biophys Acta. 1966 Mar 28;117(1):217–230. doi: 10.1016/0304-4165(66)90169-3. [DOI] [PubMed] [Google Scholar]
- Parsons R. G., Hogg R. W. Crystallization and characterization of the L-arabinose-binding protein of Escherichia coli B-r. J Biol Chem. 1974 Jun 10;249(11):3602–3607. [PubMed] [Google Scholar]
- Quiocho F. A., Gilliland G. L., Phillips G. N., Jr The 2.8-A resolution structure of the L-arabinose-binding protein from Escherichia coli. Polypeptide chain folding, domain similarity, and probable location of sugar-binding site. J Biol Chem. 1977 Jul 25;252(14):5142–5149. [PubMed] [Google Scholar]
- Quiocho F. A., Pflugrath J. W. The structure of D-galactose-binding protein at 4.1 A resolution looks like L-arabinose-binding protein. J Biol Chem. 1980 Jul 25;255(14):6559–6551. [PubMed] [Google Scholar]
- Schleif R. An L-arabinose binding protein and arabinose permeation in Escherichia coli. J Mol Biol. 1969 Nov 28;46(1):185–196. doi: 10.1016/0022-2836(69)90065-5. [DOI] [PubMed] [Google Scholar]
- Somack R. Complete phenylthiohydantoin amino acid analysis by high-performance liquid chromatography on ULTRASPHERE-octadecyltrimethyloxysilane. Anal Biochem. 1980 May 15;104(2):464–468. doi: 10.1016/0003-2697(80)90100-1. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971 Nov;108(2):695–704. doi: 10.1128/jb.108.2.695-704.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]



