Abstract
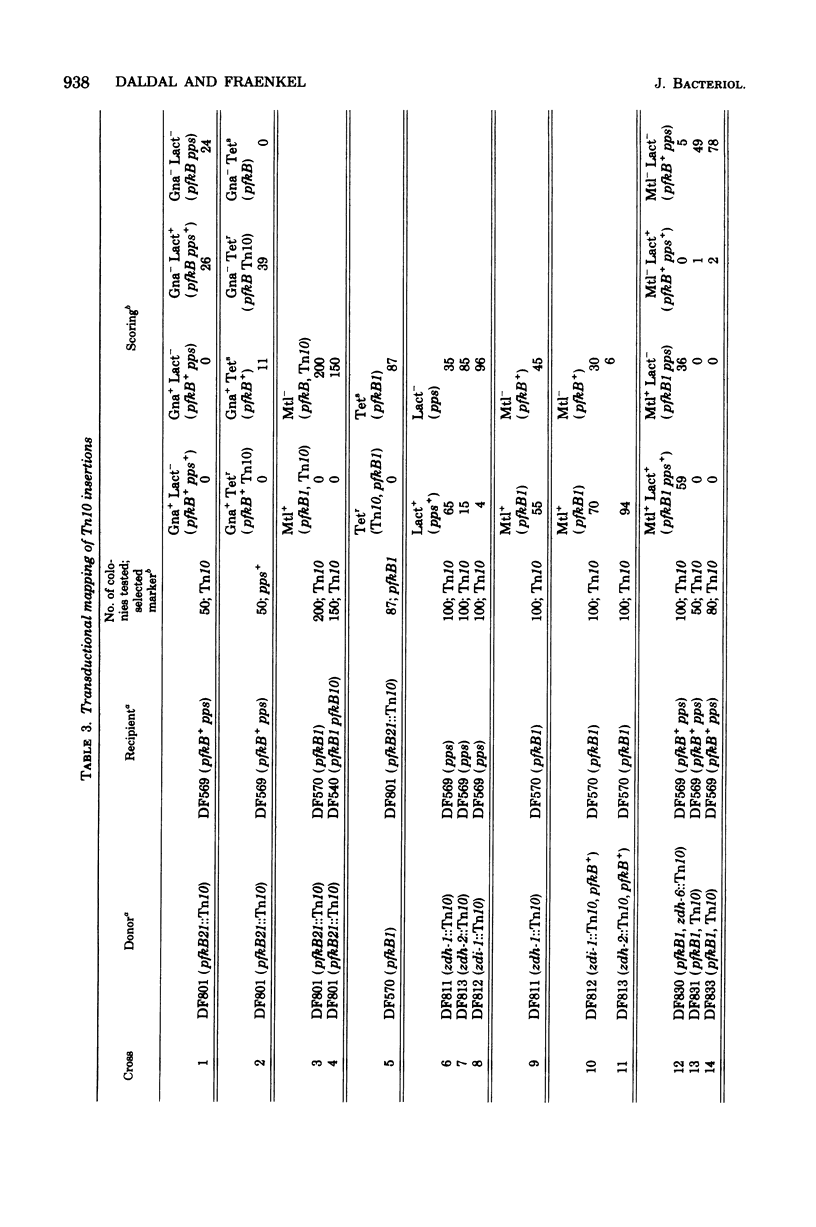
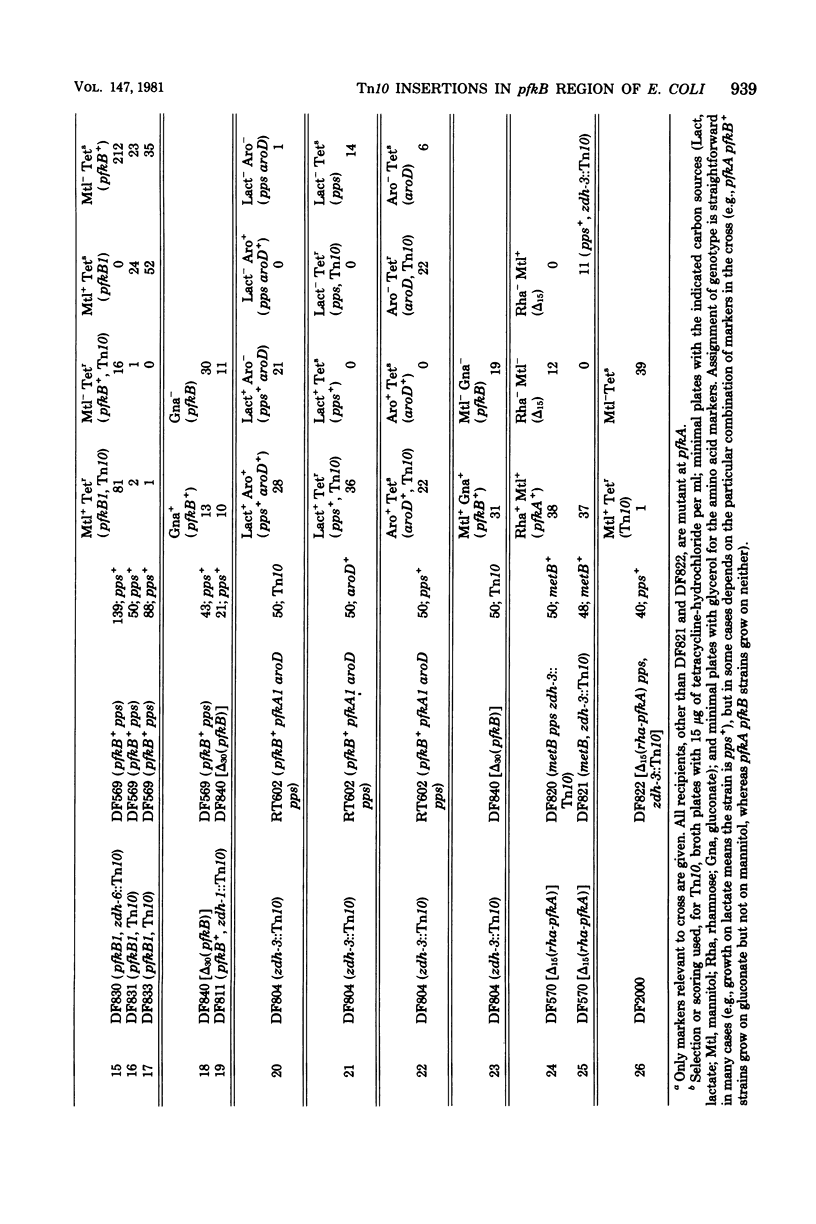
The locus pfkB is known to determine expression of a minor phosphofructokinase (Pfk-2). Pfk-2 and pfkB seem to be dispensable, since Tn10 insertions in pfkB, as well as deletions from Tn10 nearby, are obtainable. Strains deleted for both pfkA and pgkB are unable to grow at all on sugars whose primary route of metabolism is via fructose 6-phosphate, confirming earlier reports implicating the low Pfk-2 activity, rather than the pentose-phosphate pathway, as needed for the slow growth on sugars of pfkA pfkB+ strains. The pfkB locus probably contains the structural gene for Pfk-2, since a mutation closely linked to pfkB1, which affects growth on glycerol, is found to alter the enzyme. Partial phenotypic suppression of the pfkA mutant phenotype results from Tn10 insertion very close to the pps gene, ca. 0.5 min from pgkB. The insertion does not clearly affect either Pfk-2 or phosphoenolpyruvate synthetase, and the mechanism of suppression is unclear.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babul J. Phosphofructokinases from Escherichia coli. Purification and characterization of the nonallosteric isozyme. J Biol Chem. 1978 Jun 25;253(12):4350–4355. [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy D., Buc H., Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J Mol Biol. 1968 Jan 14;31(1):13–35. doi: 10.1016/0022-2836(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Location of a gene specifying phosphopyruvate synthase activity on the genome of Escherichia coli, K12. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):281–292. doi: 10.1098/rspb.1967.0066. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Chulavatnatol M., Atkinson D. E. Phosphoenolpyruvate synthetase from Escherichia coli. Effects of adenylate energy charge and modifier concentrations. J Biol Chem. 1973 Apr 25;248(8):2712–2715. [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer M. M. Gene organization around the phenylalanyl-transfer ribonucleic acid synthetase locus in Escherichia coli. J Bacteriol. 1981 Apr;146(1):269–274. doi: 10.1128/jb.146.1.269-274.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Selection of Escherichia coli mutants lacking glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase. J Bacteriol. 1968 Apr;95(4):1267–1271. doi: 10.1128/jb.95.4.1267-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Role of phosphofructokinase in the utilization of glucose by Escherichia coli. Nature. 1970 Jul 4;227(5253):44–46. doi: 10.1038/227044a0. [DOI] [PubMed] [Google Scholar]
- Kotlarz D., Buc H. Two Escherichia coli fructose-6-phosphate kinases. Preparative purification, oligomeric structure and immunological studies. Biochim Biophys Acta. 1977 Sep 15;484(1):35–48. doi: 10.1016/0005-2744(77)90111-5. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Suppressor of phosphofructokinase mutations of Escherichia coli. J Bacteriol. 1972 Oct;112(1):183–187. doi: 10.1128/jb.112.1.183-187.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narindrasorasak S., Bridger W. A. Phosphoenolypyruvate synthetase of Escherichia coli: molecular weight, subunit composition, and identification of phosphohistidine in phosphoenzyme intermediate. J Biol Chem. 1977 May 25;252(10):3121–3127. [PubMed] [Google Scholar]
- Pahel G., Bloom F. R., Tyler B. Deletion mapping of the polA-metB region of the Escherichia coli chromosome. J Bacteriol. 1979 May;138(2):653–656. doi: 10.1128/jb.138.2.653-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Benz E. J., Jr, St Peter D. A., Krieger J. N., Meuth M., Trieshmann H. W., Jr Hyperproduction and purification of nicotinamide deamidase, a microconstitutive enzyme of Escherichia coli. J Biol Chem. 1971 Nov 25;246(22):6792–6796. [PubMed] [Google Scholar]
- Robinson J. P., Fraenkel D. G. Allosteric and non-allosteric E. coli phosphofructokinases: effects on growth. Biochem Biophys Res Commun. 1978 Apr 14;81(3):858–863. doi: 10.1016/0006-291x(78)91430-4. [DOI] [PubMed] [Google Scholar]
- Roehl R. A., Vinopal R. T. Lack of glucose phosphotransferase function in phosphofructokinase mutants of Escherichia coli. J Bacteriol. 1976 May;126(2):852–860. doi: 10.1128/jb.126.2.852-860.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. G., Weiss B. Exonuclease III of Escherichia coli K-12, an AP endonuclease. Methods Enzymol. 1980;65(1):201–211. doi: 10.1016/s0076-6879(80)65028-9. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Genetic organization of the E. coli chromosome around the structural gene for initiation factor IF3 (infC). Mol Gen Genet. 1979 Feb 1;169(3):337–343. doi: 10.1007/BF00382279. [DOI] [PubMed] [Google Scholar]
- Thomson J. A. E. coli phosphofructokinase synthesized in vitro from a ColE1 hybrid plasmid. Gene. 1977 Jul;1(5-6):347–356. doi: 10.1016/0378-1119(77)90039-7. [DOI] [PubMed] [Google Scholar]
- Thomson J., Gerstenberger P. D., Goldberg D. E., Gociar E., Orozco de Silva A., Fraenkel D. G. ColE1 hybrid plasmids for Escherichia coli genes of glycolysis and the hexose monophosphate shunt. J Bacteriol. 1979 Jan;137(1):502–506. doi: 10.1128/jb.137.1.502-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Clifton D., Fraenkel D. G. PfkA locus of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1162–1171. doi: 10.1128/jb.122.3.1162-1171.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Clifton D., Fraenkel D. G. PfkA locus of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1162–1171. doi: 10.1128/jb.122.3.1162-1171.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Fraenkel D. G. Phenotypic suppression of phosphofructokinase mutations in Escherichia coli by constitutive expression of the glyoxylate shunt. J Bacteriol. 1974 Jun;118(3):1090–1100. doi: 10.1128/jb.118.3.1090-1100.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Hillman J. D., Schulman H., Reznikoff W. S., Fraenkel D. G. New phosphoglucose isomerase mutants of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1172–1174. doi: 10.1128/jb.122.3.1172-1174.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. J., Hochhauser S. J., Cintron N. M., Weiss B. Genetic mapping of xthA, the structural gene for exonuclease III in Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1082–1088. doi: 10.1128/jb.126.3.1082-1088.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



