Abstract
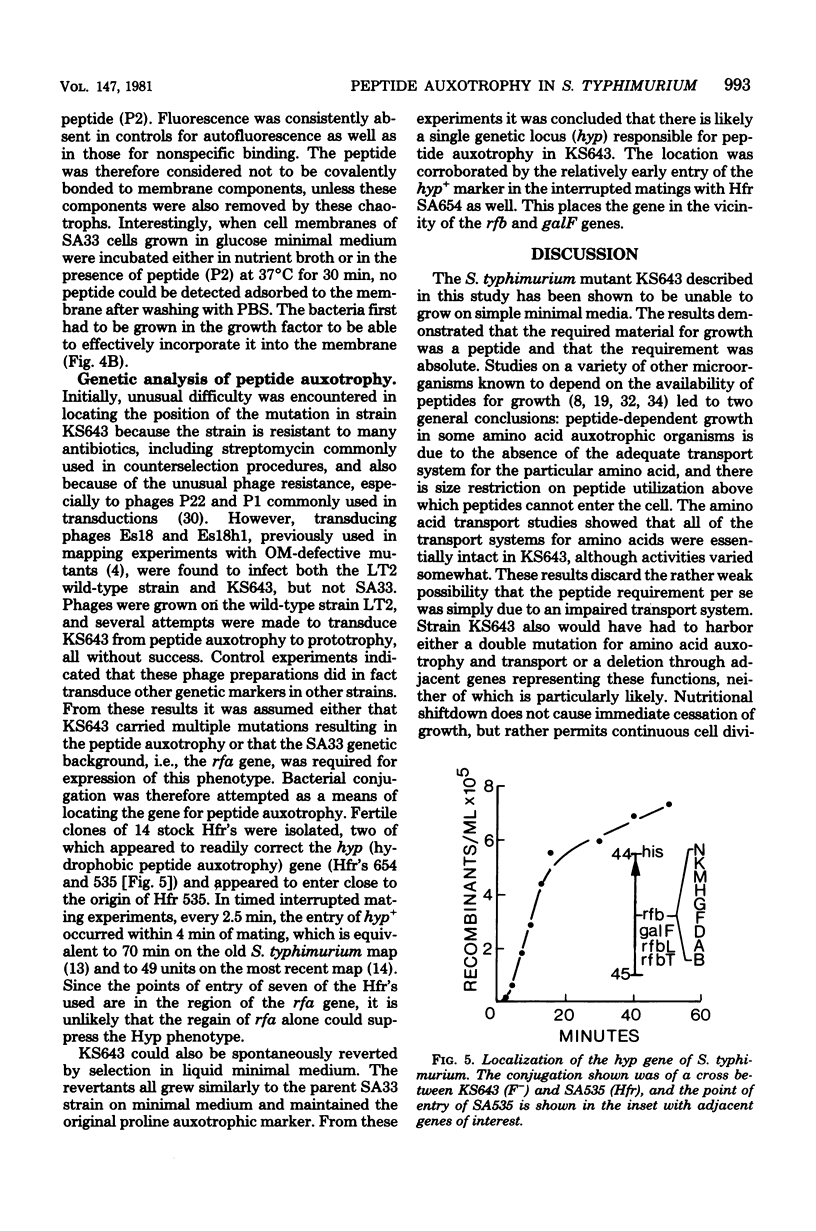
The growth of a pleiotropic membrane mutant of Salmonella typhimurium with modified lipopolysaccharide composition was found to be strictly dependent on the peptone component of complex media. Nutritional Shiftdown into minimal media allowed growth for three to four generations. Of 20 commercial peptones, only enzymatic digests supported growth to varying degrees. Neither trace cations, amino acids, vitamins, carbohydrates, lipids, glutathione, polyamines, carbodimides, nor synthetic peptides stimulated growth; however, cells still metabolized carbohydrates, and amino acid transport systems were shown to be functional. A tryptic digest of casein was fractionated into four electrophoretically different peptide fractions of 1,000 to 1,200 molecular weight which supported growth to varying degrees. The best of these was further fractionated to two highly hydrophopic peptides. N-terminal modifications eliminated biological activity. Fluorescein-conjugated goat antibody to rabbit immunoglobulin G was used as a probe to detect antipeptide antibody-peptide complexes on membrane preparations. Cells grown on peptone distributed the peptide into both inner and outer membranes. The peptide could be removed with chaotropic agents, and cells had to be pregrown in peptone-containing media to bind the hydrophobic peptide. The gene (hyp) responsible for peptide auxotrophy was mapped at 44 to 45 units by conjugation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Ames G. F., Young J. D., Tsuchiya D., Lecocq J. Illicit transport: the oligopeptide permease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):456–458. doi: 10.1073/pnas.70.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón D. N. Positive selection of mutants with cell envelope defects of a Salmonells typhimurium strain hypersensitive to the products of genes hisF and hisH. J Bacteriol. 1979 Mar;137(3):1271–1281. doi: 10.1128/jb.137.3.1271-1281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Bosch V., Klumpp E. R., Neff I., Mayer H., Schlecht S. Antigenic determinants of murein lipoprotein and its exposure at the surface of Enterobacteriaceae. Eur J Biochem. 1976 Mar 1;62(3):555–566. doi: 10.1111/j.1432-1033.1976.tb10190.x. [DOI] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly W. J., Barry J. G., Richardson T. 14C-Methylated beta-casein as a substrate for plasmin, and its application to the study of milk protein transformations. Biochim Biophys Acta. 1980 Nov 20;626(1):117–126. doi: 10.1016/0005-2795(80)90203-2. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Eur J Biochem. 1979 Feb 1;93(3):609–620. doi: 10.1111/j.1432-1033.1979.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Hirshfield I. N., Price M. B. Utilization of selected leucine peptide amides by Escherichia coli. J Bacteriol. 1975 Jun;122(3):966–975. doi: 10.1128/jb.122.3.966-975.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. T., Stocker B. A. Mapping of rfa Genes in Salmonella typhimurium by ES18 and P22 Transduction and by Conjugation. J Bacteriol. 1972 Oct;112(1):48–57. doi: 10.1128/jb.112.1.48-57.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Role of a major outer membrane protein in Escherichia coli. J Bacteriol. 1977 Aug;131(2):631–637. doi: 10.1128/jb.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. Annu Rev Microbiol. 1975;29:485–504. doi: 10.1146/annurev.mi.29.100175.002413. [DOI] [PubMed] [Google Scholar]
- Myers D. E., Stocker B. A., Roantree R. J. Mapping of genes determining penicillin-resistance and serum-sensitivity in Salmonella enteritidis. J Gen Microbiol. 1980 Jun;118(2):367–376. doi: 10.1099/00221287-118-2-367. [DOI] [PubMed] [Google Scholar]
- Naider F., Becker J. M., Katzir-Katchalski E. Utilization of methionine-containing peptides and their derivatives by a methionine-requiring auxotroph of Saccharomyces cerevisiae. J Biol Chem. 1974 Jan 10;249(1):9–20. [PubMed] [Google Scholar]
- Naider F., Becker J. M. Multiplicity of oligopeptide transport systems in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1208–1215. doi: 10.1128/jb.122.3.1208-1215.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Multiple molecular forms of uridine diphosphate glucose pyrophosphorylase from Salmonella typhimurium. I. Catalytic properties of various forms. J Biol Chem. 1971 Jul 25;246(14):4386–4396. [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Multiple molecular forms of uridine diphosphate glucose pyrophosphorylase from Salmonella typhimurium. II. Genetic determination of multiple forms. J Biol Chem. 1971 Jul 25;246(14):4397–4403. [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Cordaro J. C., Roseman S. Sugar transport. A pleiotropic membrane mutant of Salmonella typhimurium. J Biol Chem. 1977 Nov 10;252(21):7862–7876. [PubMed] [Google Scholar]
- Rottem S., Leive L. Effect of variations in lipopolysaccharide on the fluidity of the outer membrane of Escherichia coli. J Biol Chem. 1977 Mar 25;252(6):2077–2081. [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Langworthy T. A., Smith M. R. Polypeptide nature of growth requirement in yeast extract for Thermoplasma acidophilum. J Bacteriol. 1975 Nov;124(2):884–892. doi: 10.1128/jb.124.2.884-892.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij A., van Alphen L., Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli K12. II. Freeze fracture morphology of wild type and mutant strains. Biochim Biophys Acta. 1977 Apr 18;466(2):269–282. doi: 10.1016/0005-2736(77)90224-3. [DOI] [PubMed] [Google Scholar]
- Wahren A., Holme T. Amino acid and peptide requirement of Fusiformis necrophorus. J Bacteriol. 1973 Oct;116(1):279–284. doi: 10.1128/jb.116.1.279-284.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Arai T., Hattori T. Effects of cell wall polysaccharide on the mating ability of Salmonella typhimurium. Nature. 1970 Jan 3;225(5227):70–71. doi: 10.1038/225070a0. [DOI] [PubMed] [Google Scholar]
- Wolfinbarger L., Jr, Marzluf G. A. Size restriction on utilization of peptides by amino acid auxotrophs of Neurospora crassa. J Bacteriol. 1975 Jun;122(3):949–956. doi: 10.1128/jb.122.3.949-956.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C., Green L., Miller C. G. Degradation of intracellular protein in Salmonella typhimurium peptidase mutants. J Mol Biol. 1980 Oct 15;143(1):21–33. doi: 10.1016/0022-2836(80)90122-9. [DOI] [PubMed] [Google Scholar]




