Abstract
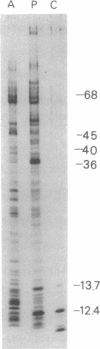
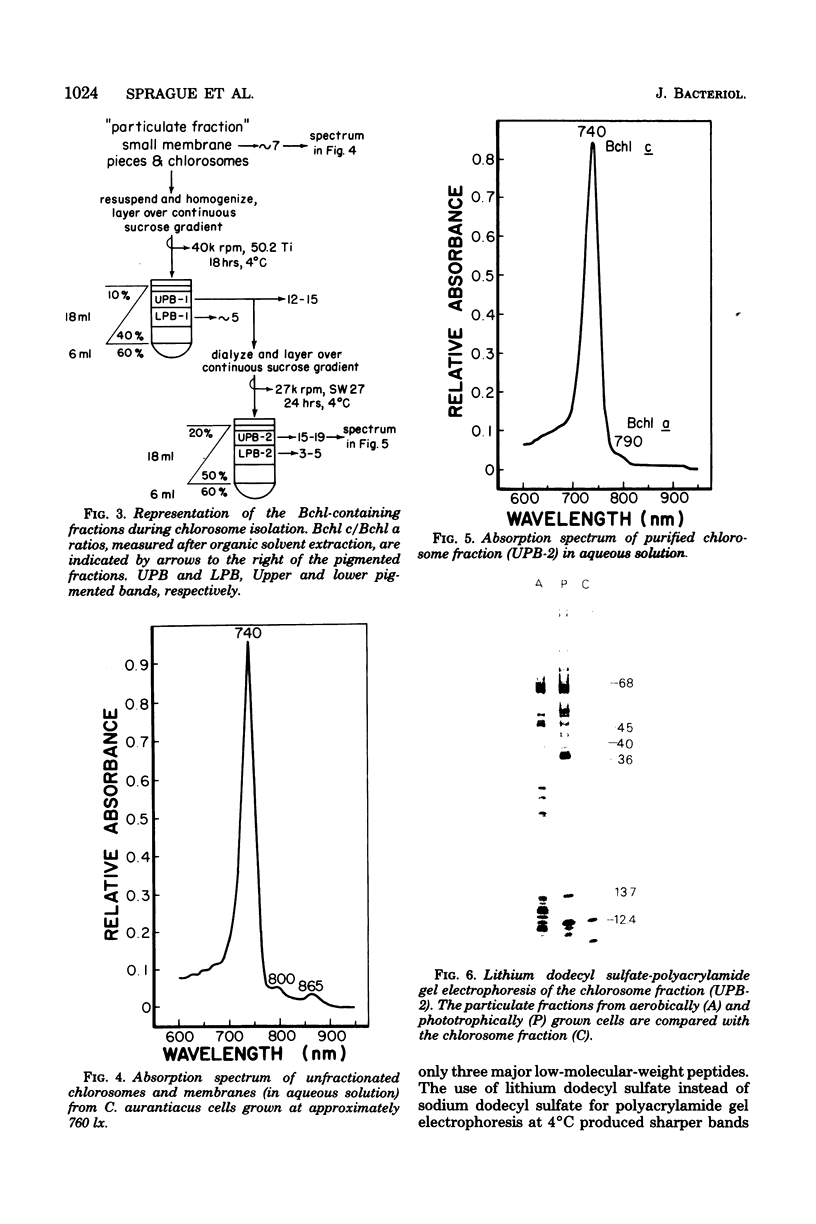
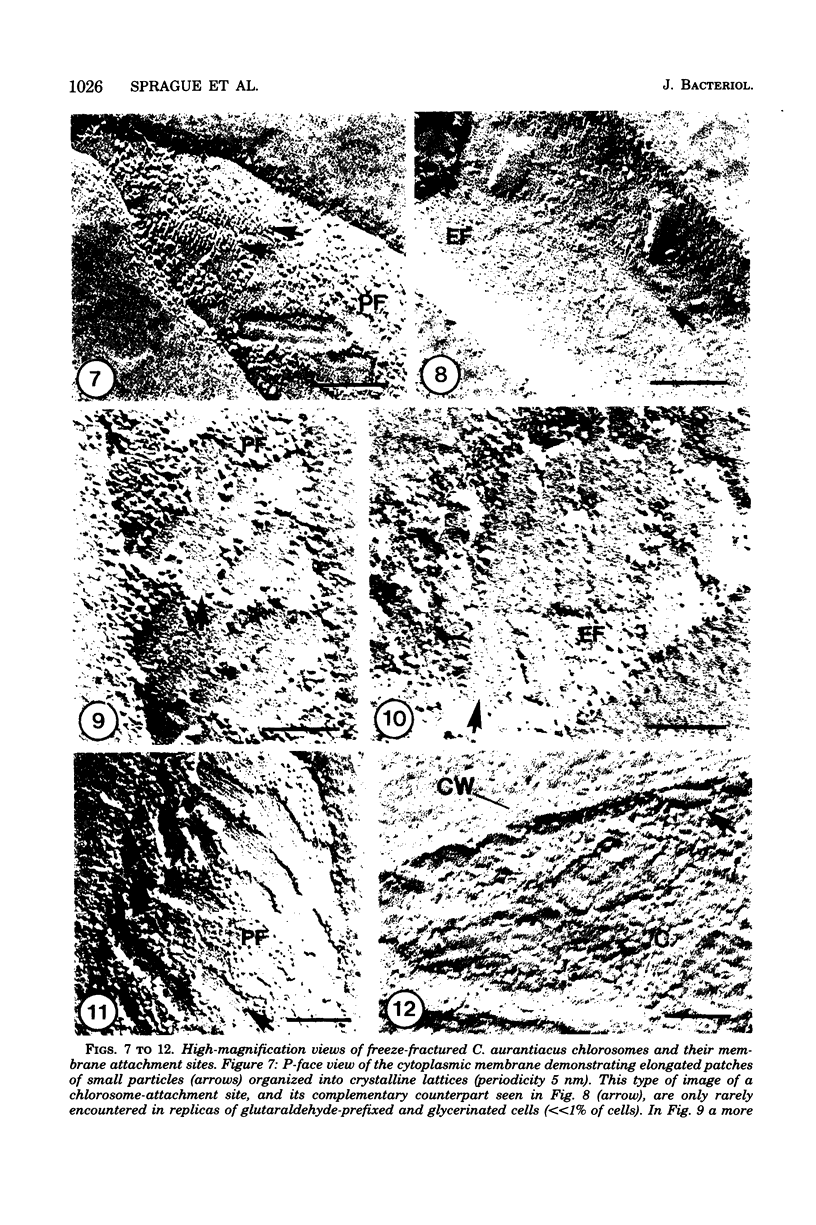
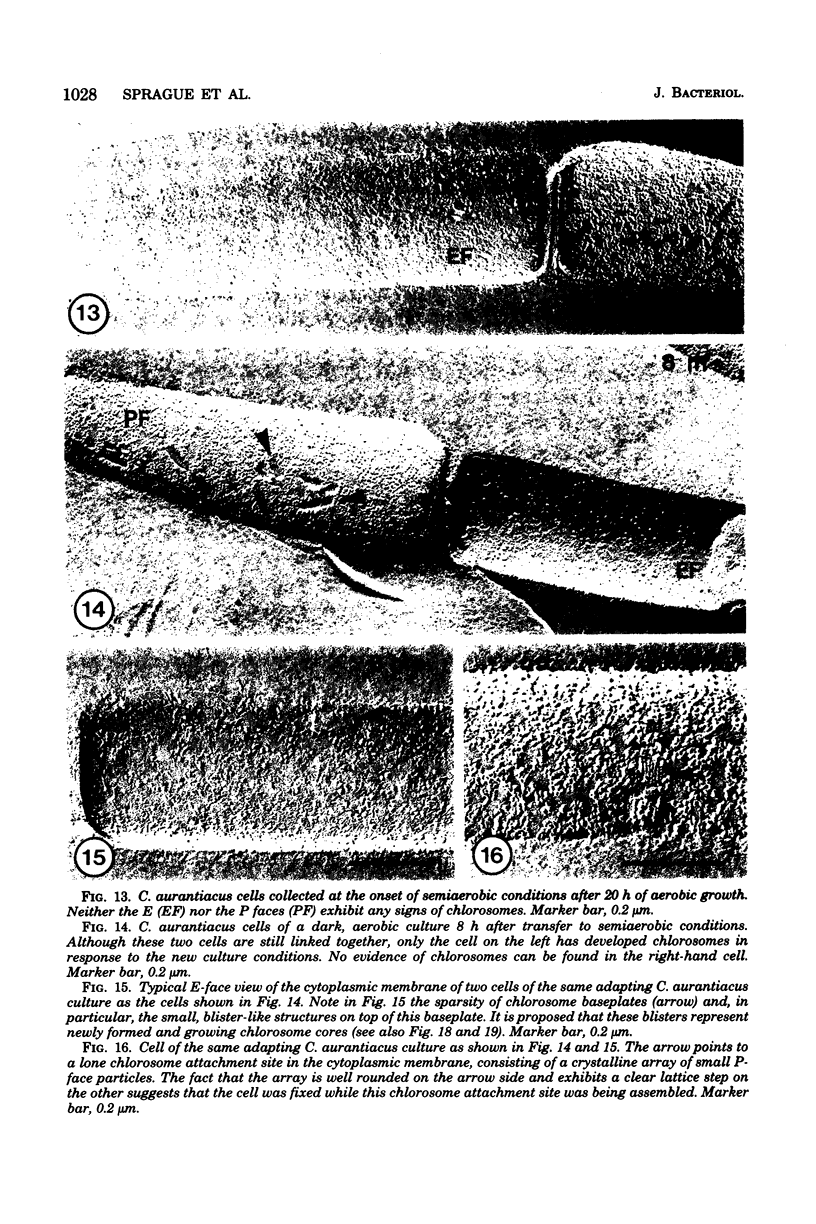
Freeze-fracture electron microscopy was used to study further the changes in chlorosome structure during the development of the photosynthetic apparatus in Chloroflexus aurantiacus J-10-fl. During development, in response to decreased light intensity or lower oxygen tension, the number of chlorosomes per cell increased. The same conditions also led to a general thickening of chlorosomes but did not affect their length or width. The thickening of the chlorosomes paralleled increases in the bacteriochlorophyll c/bacteriochlorophyll a ratio. Semiaerobic induction of the photosynthetic apparatus did not produce a synchronous assembly of chlorosomes in all cells of a given culture. Even adjacent cells of a single filament showed great variations in the rate and extent of response. Parallel appearance of (i) approximately 5-nm particles (in a lattice configuration) in the membrane attachment site, (ii) the crystalline baseplate material (with a periodicity of approximately 6 nm) adjacent to the membrane attachment site, and (iii) the chlorosome envelope layer preceded addition of longitudinally oriented, rodlike elements (diameter, congruent to 6 m) to the chlorosome core. It is estimated that each chlorosome can funnel energy into approximately 100 reaction centers. Chlorosomes could be isolated by a simple density gradient procedure only from cells grown at low light intensity. A bacteriochlorophyll a species absorbing at 790 nm was associated with isolated chlorosomes. Lithium dodecyl sulfate-polyacrylamide gel electrophoresis of chlorosomes showed only a few low-molecular-weight polypeptides (less than 15,000).
Full text
PDF




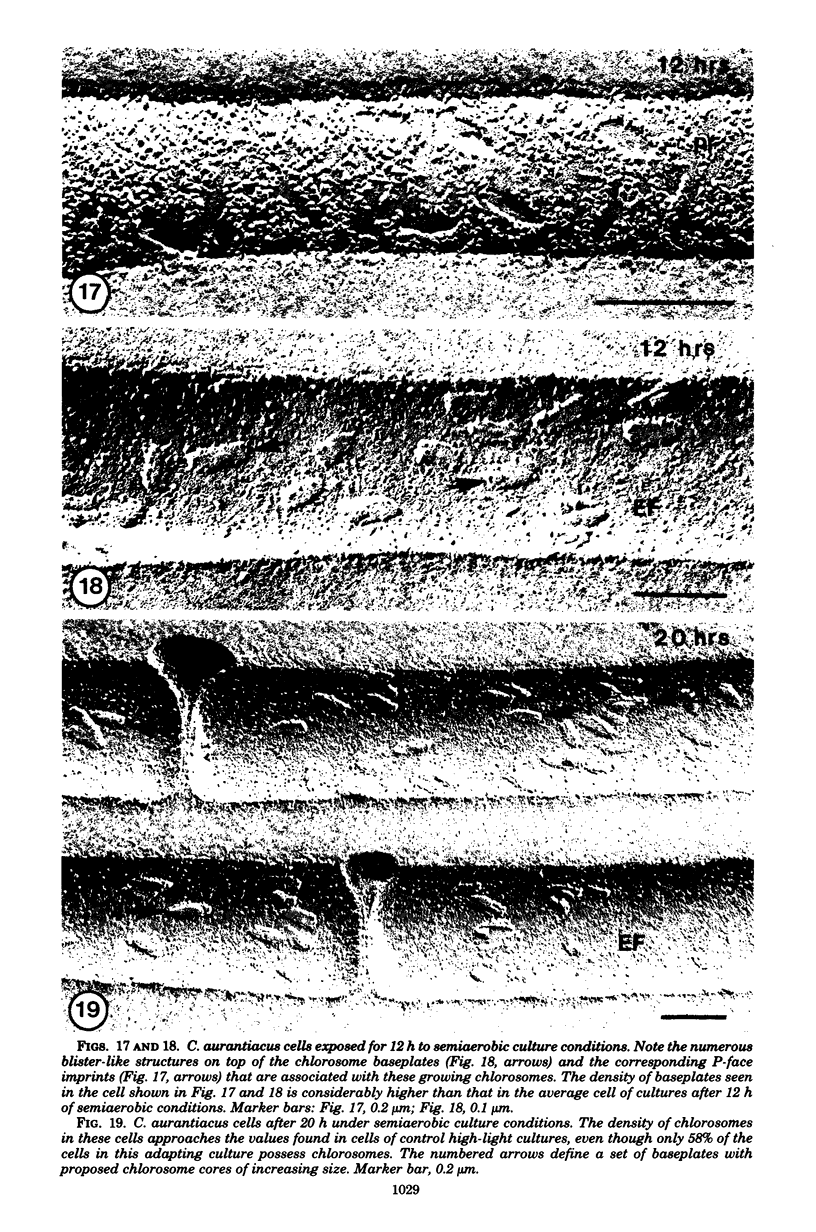


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Broch-Due M., Ormerod J. G., Fjerdingen B. S. Effect of light intensity of vesicle formation in chlorobium. Arch Microbiol. 1978 Mar;116(3):269–274. doi: 10.1007/BF00417850. [DOI] [PubMed] [Google Scholar]
- Broglie R. M., Hunter C. N., Delepelaire P., Niederman R. A., Chua N. H., Clayton R. K. Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):87–91. doi: 10.1073/pnas.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., PFENNIG N., KUNISAWA R. THE FINE STRUCTURE OF GREEN BACTERIA. J Cell Biol. 1964 Jul;22:207–225. doi: 10.1083/jcb.22.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden D. L., Stanier R. Y. The characterization of chlorobium vesicles and membranes isolated from green bacteria. Arch Mikrobiol. 1970;72(2):115–134. doi: 10.1007/BF00409518. [DOI] [PubMed] [Google Scholar]
- Fowler C. F., Nugent N. A., Fuller R. C. The isolation and characterization of a photochemically active complex from Chloropseudomonas ethylica. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2278–2282. doi: 10.1073/pnas.68.9.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Conti S. F., Fuller R. C. Effect of light intensity on the formation of the photochemical apparatus in the green bacterium Chloropseudomonas ethylicum. J Bacteriol. 1966 Jan;91(1):349–355. doi: 10.1128/jb.91.1.349-355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Conti S. F., Fuller R. C. Photosynthetic Apparatus in the Green Bacterium Chloropseudomonas ethylicum. J Bacteriol. 1966 Jan;91(1):311–323. doi: 10.1128/jb.91.1.311-323.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W., Fenna R. E., Bolognesi M. C., Schmid M. F., Olson J. M. Structure of a bacteriochlorophyll a-protein from the green photosynthetic bacterium Prosthecochloris aestuarii. J Mol Biol. 1979 Jun 25;131(2):259–285. doi: 10.1016/0022-2836(79)90076-7. [DOI] [PubMed] [Google Scholar]
- Olson J. M., Prince R. C., Brune D. C. Reaction-center complexes from green bacteria. Brookhaven Symp Biol. 1976 Jun 7;(28):238–246. [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol. 1974;100(1):5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., SMITH J. H. The chlorophylis of green bacteria. Biochim Biophys Acta. 1960 Jul 15;41:478–484. doi: 10.1016/0006-3002(60)90045-7. [DOI] [PubMed] [Google Scholar]
- Sprague S. G., Staehelin L. A., Fuller R. C. Semiaerobic induction of bacteriochlorophyll synthesis in the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1981 Sep;147(3):1032–1039. doi: 10.1128/jb.147.3.1032-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]