Abstract
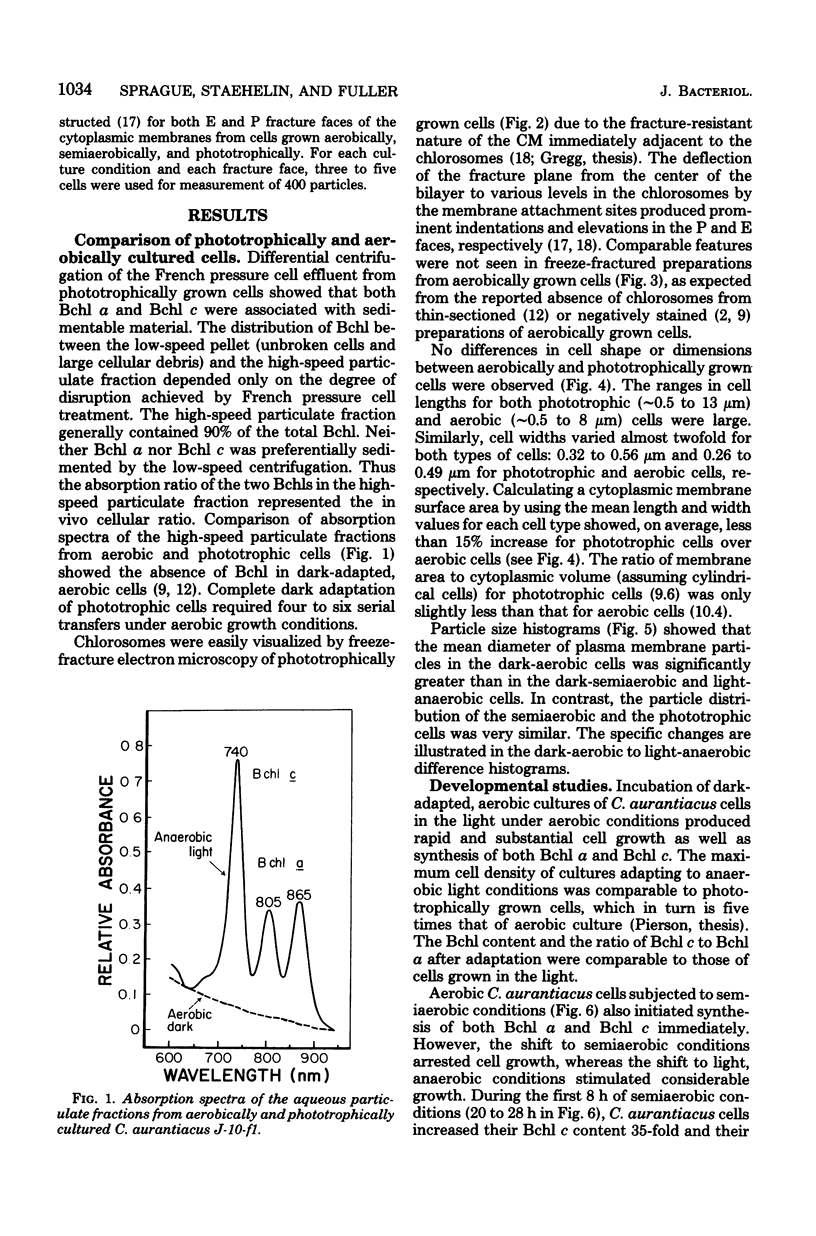
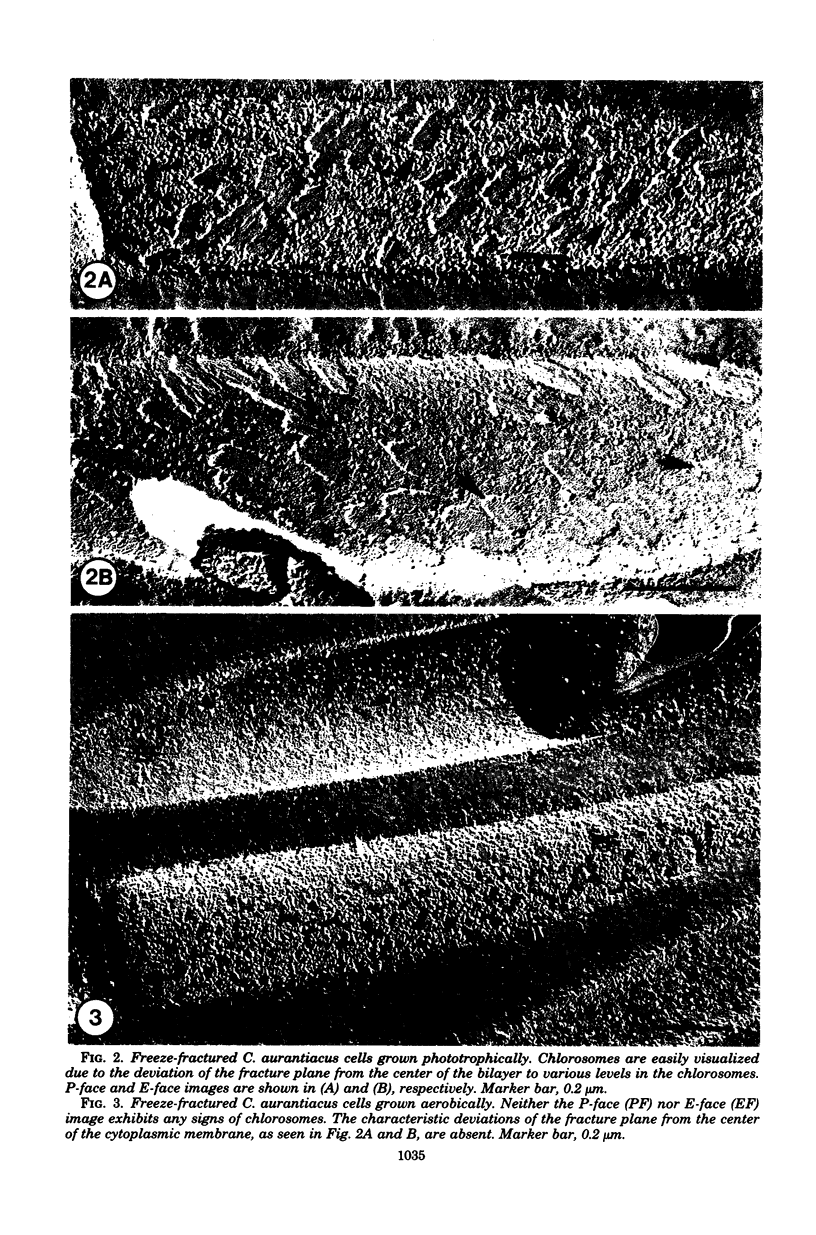
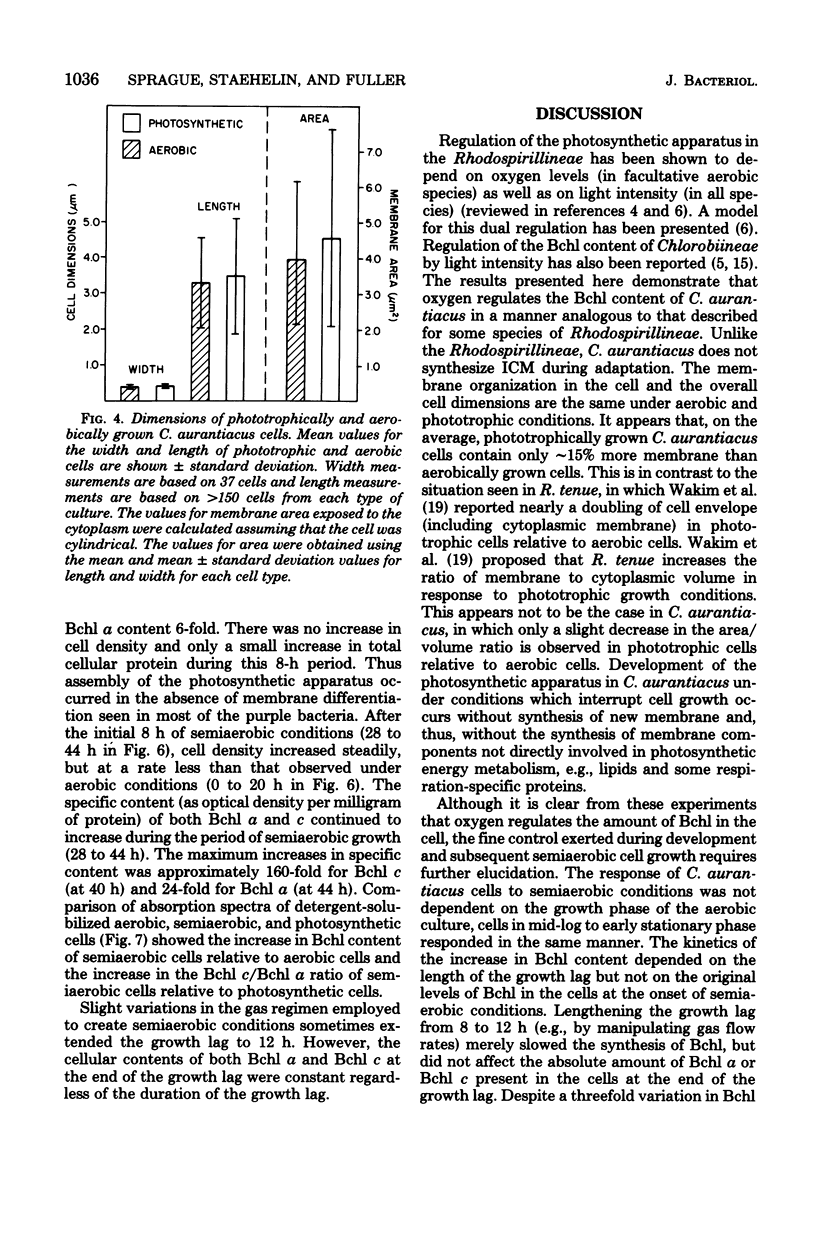
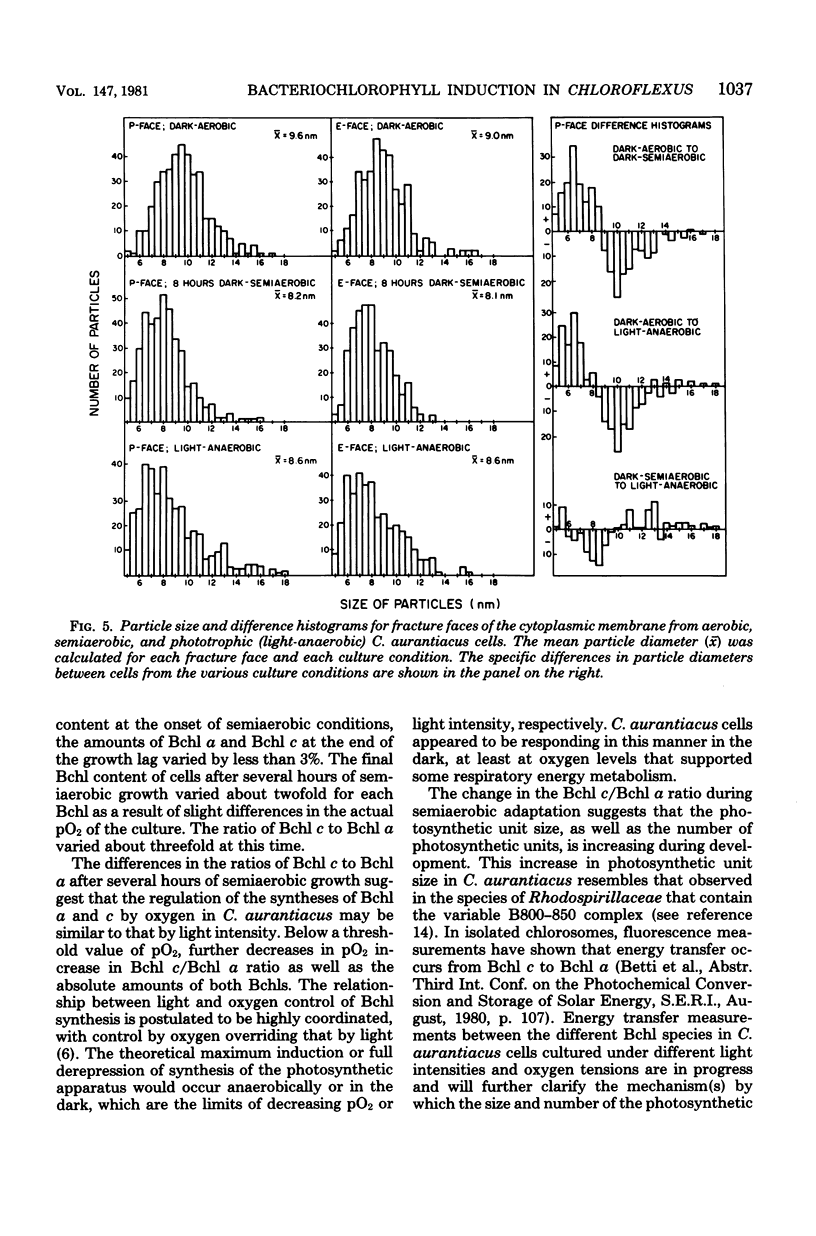
Comparison of Chloroflexus aurantiacus J-10-fl cells by freeze-fracture electron microscopy showed that cell shape and dimensions did not depend on oxygen tension or light intensity during growth. The major morphological difference between cells cultured anaerobically in the light and aerobically in the dark was the absence of chlorosomes in aerobically grown cells. C. aurantiacus cells cultured aerobically in the dark began bacteriochlorophyll synthesis immediately when shifted to either phototrophic or semiaerobic conditions. Cells adapting to phototrophic conditions grew to the same density and synthesized as much bacteriochlorophyll as nonadapting phototrophic cultures grown at the same light intensity. Cells adapting to reduced oxygen tension (semiaerobic conditions) in the dark entered an 8- to 12-h growth lag during which the bacteriochlorophyll content increased significantly. Despite variations in the initial bacteriochlorophyll content and in the length of the growth lag, the amounts of bacteriochlorophyll a and c were constant at the end of the semiaerobic growth lag. At later times during adaptation to semiaerobic conditions, after growth resumed, variations in the ratio of bacteriochlorophyll c/bacteriochlorophyll a were observed and suggested independent regulation of the two bacteriochlorophylls.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Castenholz R. W. Thermophilic blue-green algae and the thermal environment. Bacteriol Rev. 1969 Dec;33(4):476–504. doi: 10.1128/br.33.4.476-504.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Conti S. F., Fuller R. C. Effect of light intensity on the formation of the photochemical apparatus in the green bacterium Chloropseudomonas ethylicum. J Bacteriol. 1966 Jan;91(1):349–355. doi: 10.1128/jb.91.1.349-355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madigan M. T., Brock T. D. Photosynthetic sulfide oxidation by Chloroflexus aurantiacus, a filamentous, photosynthetic, gliding bacterium. J Bacteriol. 1975 May;122(2):782–784. doi: 10.1128/jb.122.2.782-784.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson R. E. Clinical nutrition, an interface between human ecology and internal medicine. Nutr Rev. 1978 Jun;36(6):161–178. doi: 10.1111/j.1753-4887.1978.tb03745.x. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol. 1974;100(1):5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- Sprague S. G., Staehelin L. A., DiBartolomeis M. J., Fuller R. C. Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1981 Sep;147(3):1021–1031. doi: 10.1128/jb.147.3.1021-1031.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Golecki J. R., Drews G. Supramolecular organization of chlorosomes (chlorobium vesicles) and of their membrane attachment sites in Chlorobium limicola. Biochim Biophys Acta. 1980 Jan 4;589(1):30–45. doi: 10.1016/0005-2728(80)90130-9. [DOI] [PubMed] [Google Scholar]



