Abstract
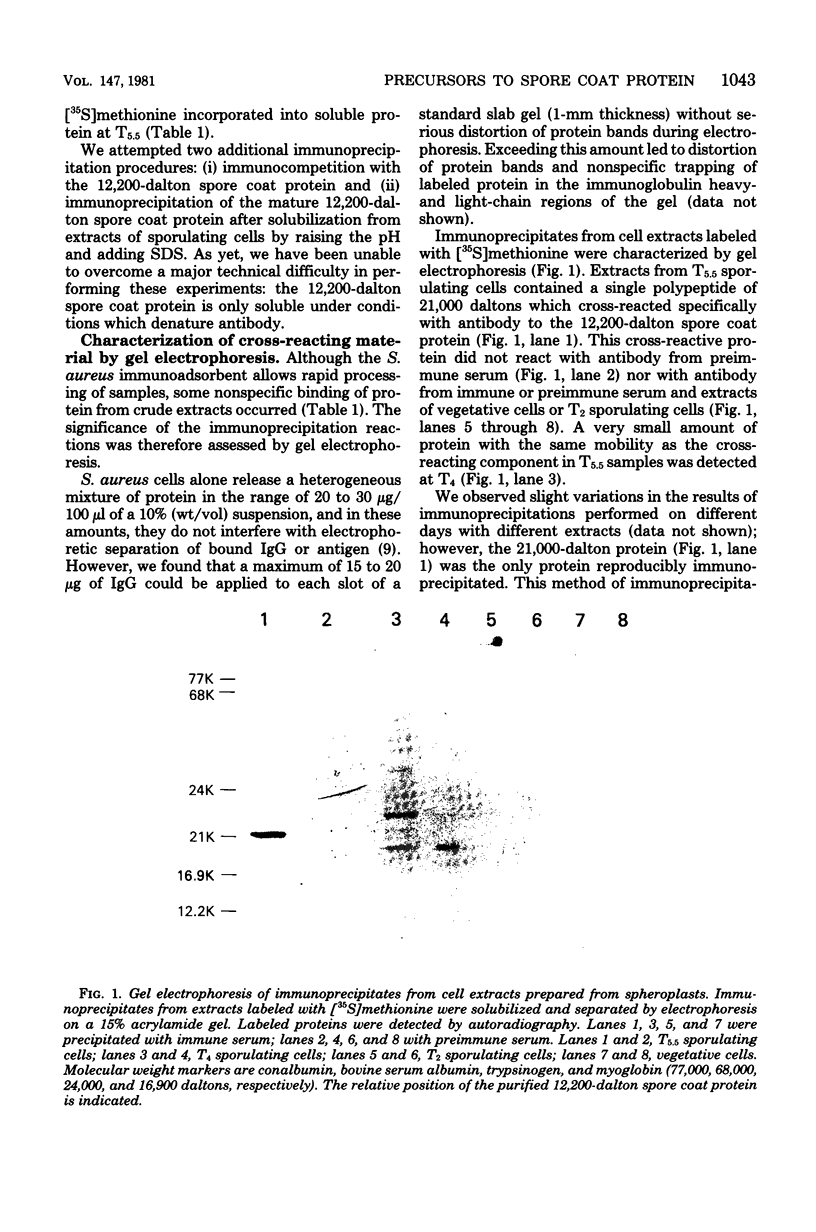
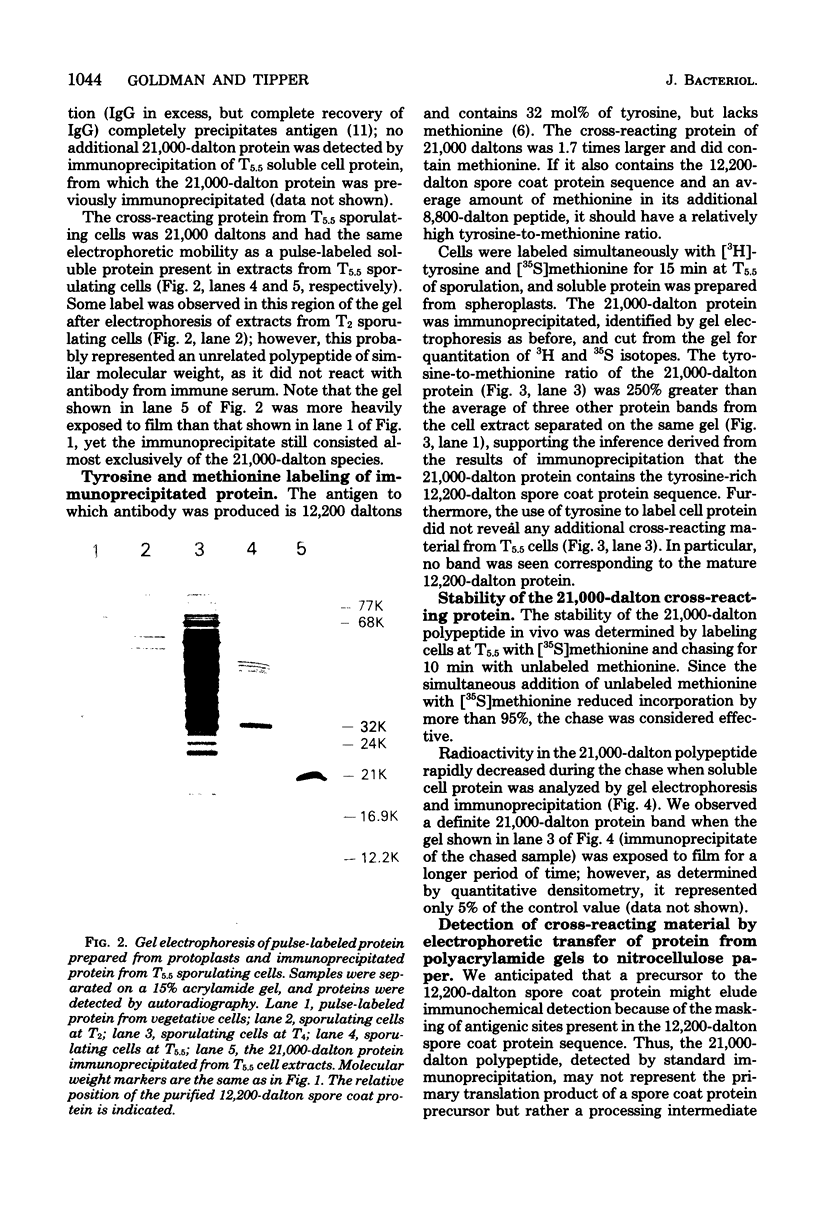
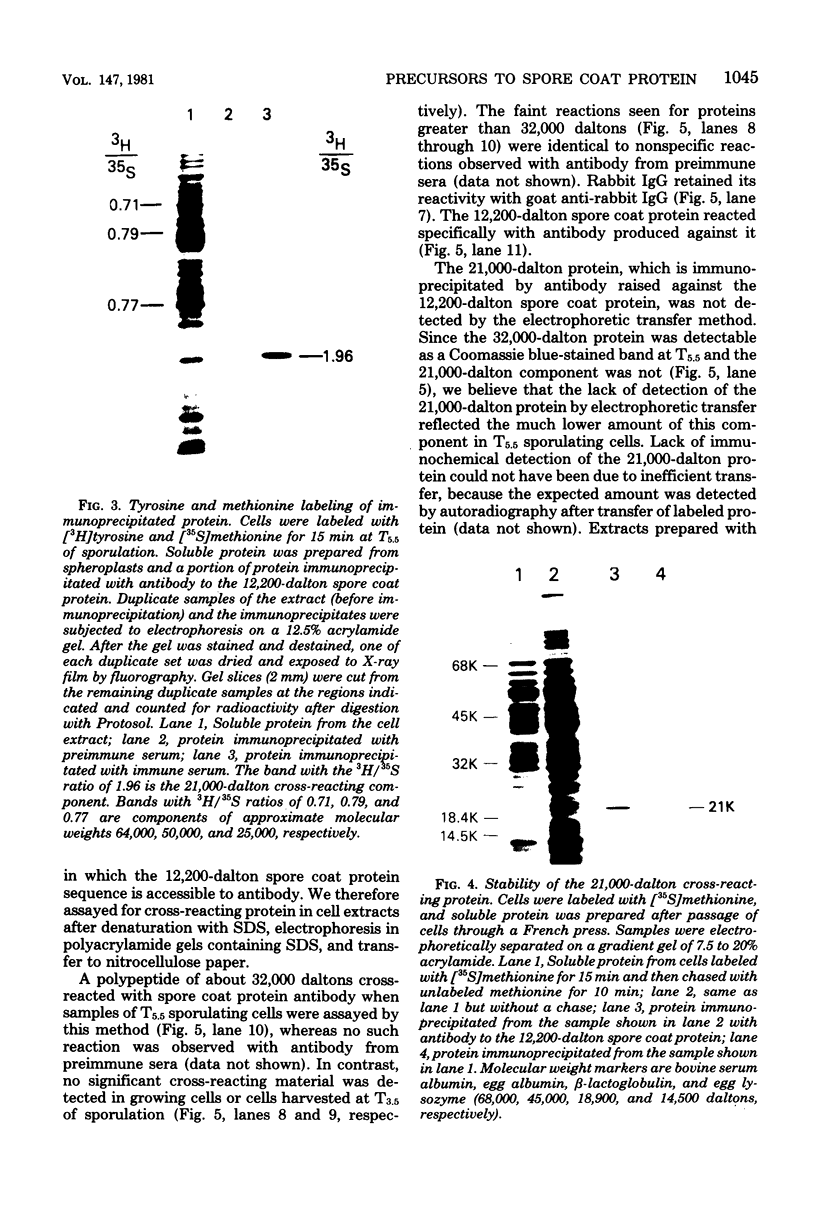
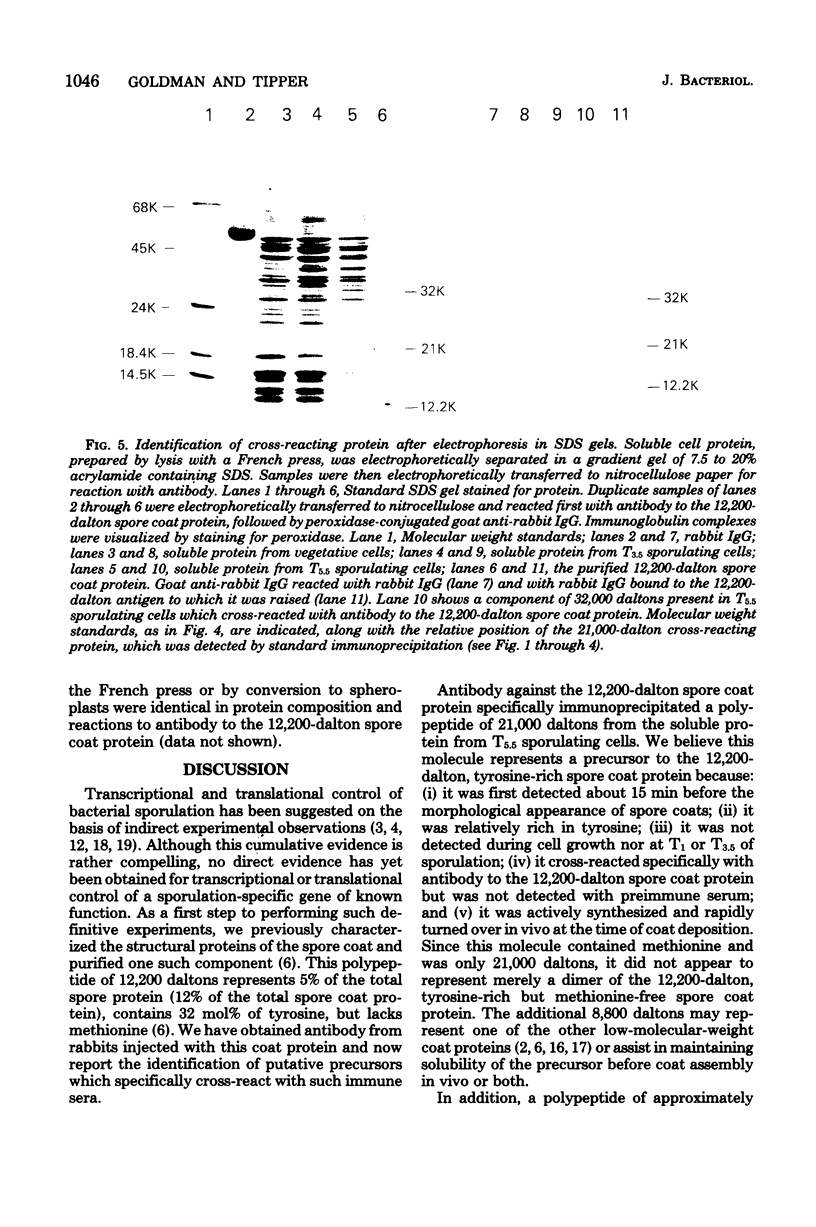
Antibody specific to the 12,200-dalton spore coat protein of Bacillus subtilis was used to detect the synthesis of cross-reacting material during sporulation. Cross-reacting protein was first detected by immunoprecipitation after 4 h of development and represented at least 1 to 2% of the total soluble protein synthesis at 5.5 h. A polypeptide of 21,000 daltons was detected in immunoprecipitates by gel electrophoresis. This polypeptide did not accumulate in sporulating cells and was rapidly turned over at the time of coat deposition. In contrast, a 32,000-dalton polypeptide reacted with antibody when unlabeled cell protein was denatured with sodium dodecyl sulfate, separated by gel electrophoresis, and transferred to nitrocellulose paper. This polypeptide was not detected during cell growth or the first 3.5 h of development but was found to accumulate in sporulating cells at 5.5 h. The lack of detection of this polypeptide by immunoprecipitation of undenatured protein indicates that the antigenic sites which cross-reacted with antibody to the 12,200-dalton protein sequence were not exposed unless the molecular conformation was altered. The 32,000-dalton protein may be a primary translation product which is proteolytically processed into mature spore coat protein via a 21,000-dalton intermediate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I. Synthesis of Bacillus cereus spore coat protein. J Bacteriol. 1981 Jan;145(1):541–547. doi: 10.1128/jb.145.1.541-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCioccio R. A., Strauss N. Patterns of transcription in Bacillus subtilis during sporulation. J Mol Biol. 1973 Jun 25;77(2):325–336. doi: 10.1016/0022-2836(73)90338-0. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Bergmann R. Alteration of the ribosomal fraction of Bacillus subtilis during sporulation. Biochim Biophys Acta. 1973 Feb 23;299(1):136–141. doi: 10.1016/0005-2787(73)90404-8. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Doi R. H. Two polypeptides associated with the ribonucleic acid polymerase core of Bacillus subtilis during sporulation. J Bacteriol. 1977 Jan;129(1):422–432. doi: 10.1128/jb.129.1.422-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Tipper D. J. Bacillus subtilis spore coats: complexity and purification of a unique polypeptide component. J Bacteriol. 1978 Sep;135(3):1091–1106. doi: 10.1128/jb.135.3.1091-1106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Losick R. A modified RNA polymerase transcribes a cloned gene under sporulation control in Bacillus subtilis. Nature. 1979 Nov 15;282(5736):256–260. doi: 10.1038/282256a0. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. Sporulation-specific translational discrimination in Bacillus subtilis. J Mol Biol. 1974 Jul 15;86(4):855–863. doi: 10.1016/0022-2836(74)90358-1. [DOI] [PubMed] [Google Scholar]
- Linn T., Greenleaf A. L., Losick R. RNA polymerase from sporulating Bacillus subtilis. Purification and properties of a modified form of the enzyme containing two sporulation polypeptides. J Biol Chem. 1975 Dec 25;250(24):9256–9261. [PubMed] [Google Scholar]
- Linn T., Losick R. The program of protein synthesis during sporulation in Bacillus subtilis. Cell. 1976 May;8(1):103–114. doi: 10.1016/0092-8674(76)90191-4. [DOI] [PubMed] [Google Scholar]
- Munoz L., Sadaie Y., Doi R. H. Spore coat protein of Bacillus subtilis. Structure and precursor synthesis. J Biol Chem. 1978 Oct 10;253(19):6694–6701. [PubMed] [Google Scholar]
- Pandey N. K., Aronson A. I. Properties of the Bacillus subtilis spore coat. J Bacteriol. 1979 Mar;137(3):1208–1218. doi: 10.1128/jb.137.3.1208-1218.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Doi R. H. Transcription from the complementary deoxyribonucleic acid strands of Bacillus subtilis during various stages of sporulation. J Bacteriol. 1974 Feb;117(2):775–782. doi: 10.1128/jb.117.2.775-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Johnson C. W., Ginther C. L., Leighton T., Wittmann H. G. Erythromycin resistant mutations in Bacillus subtilis cause temperature sensitive sporulation. Mol Gen Genet. 1977 Jan 18;150(2):147–159. doi: 10.1007/BF00695395. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]