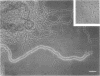
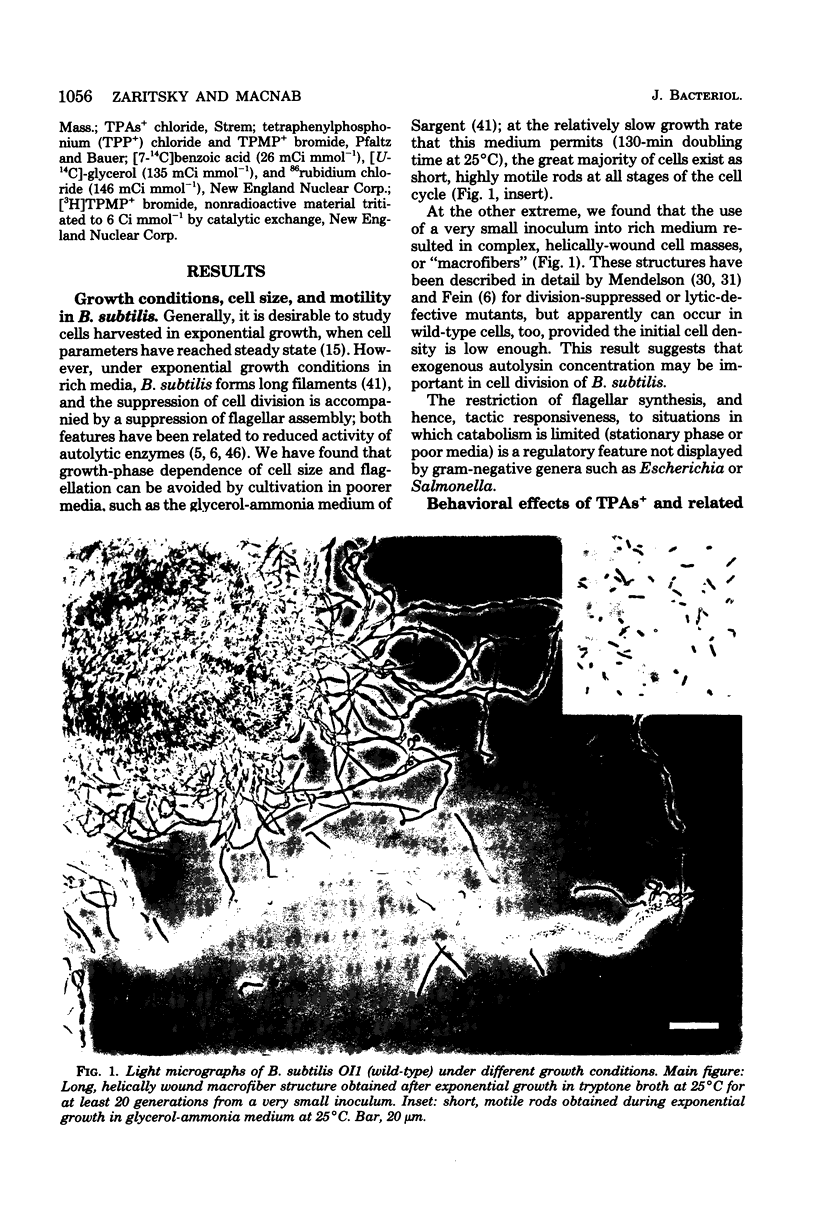
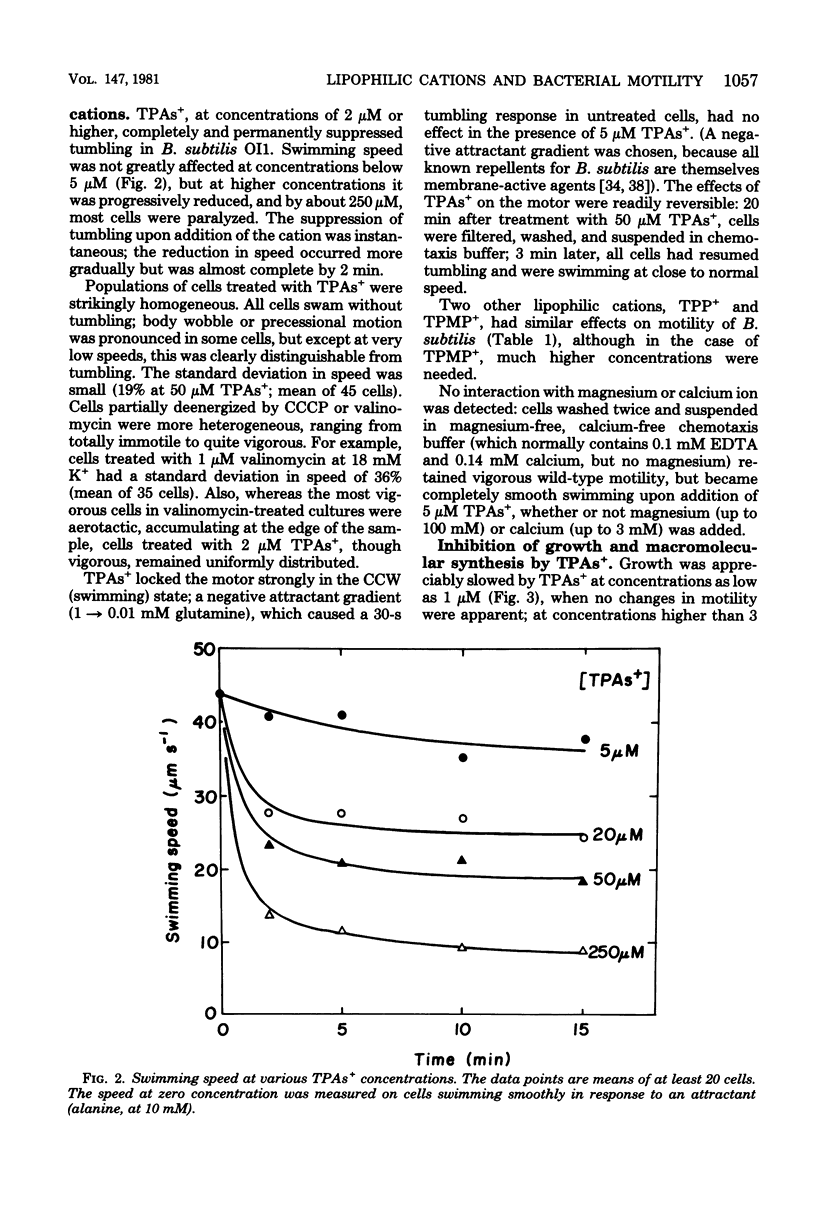
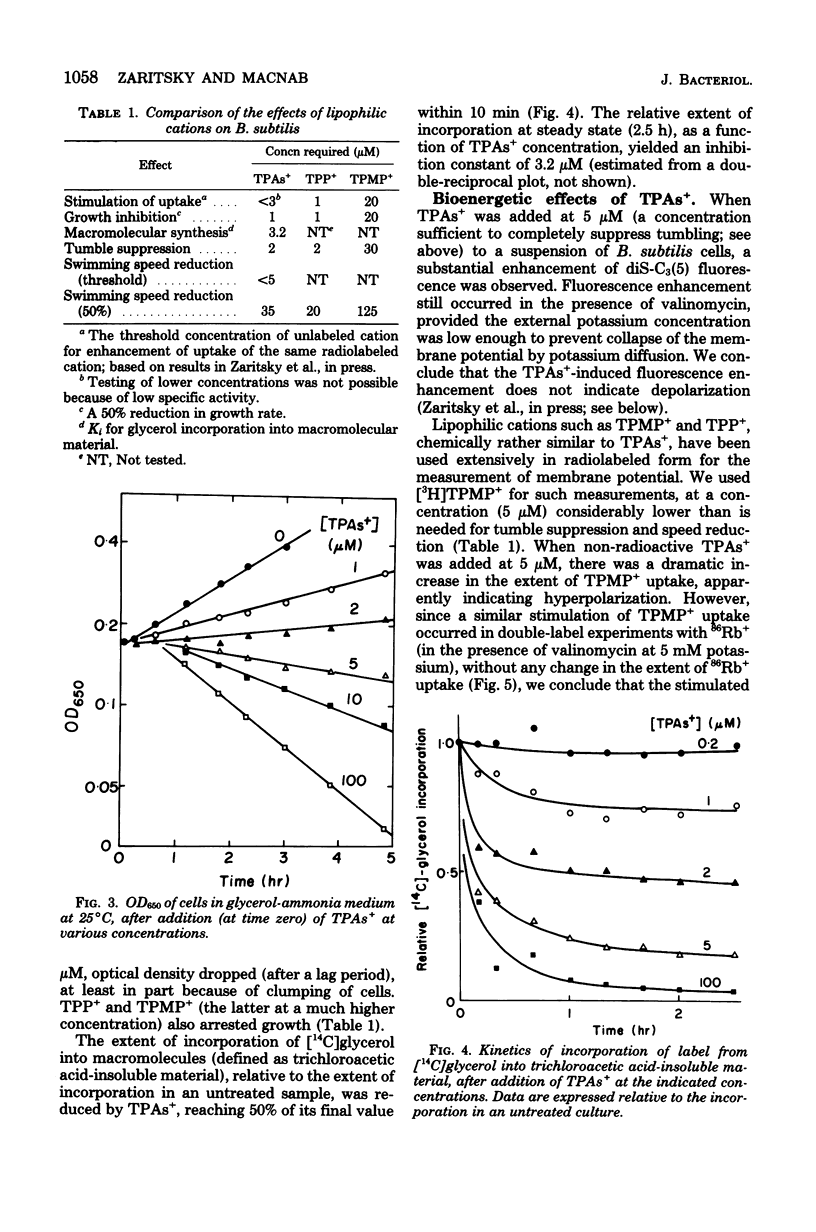
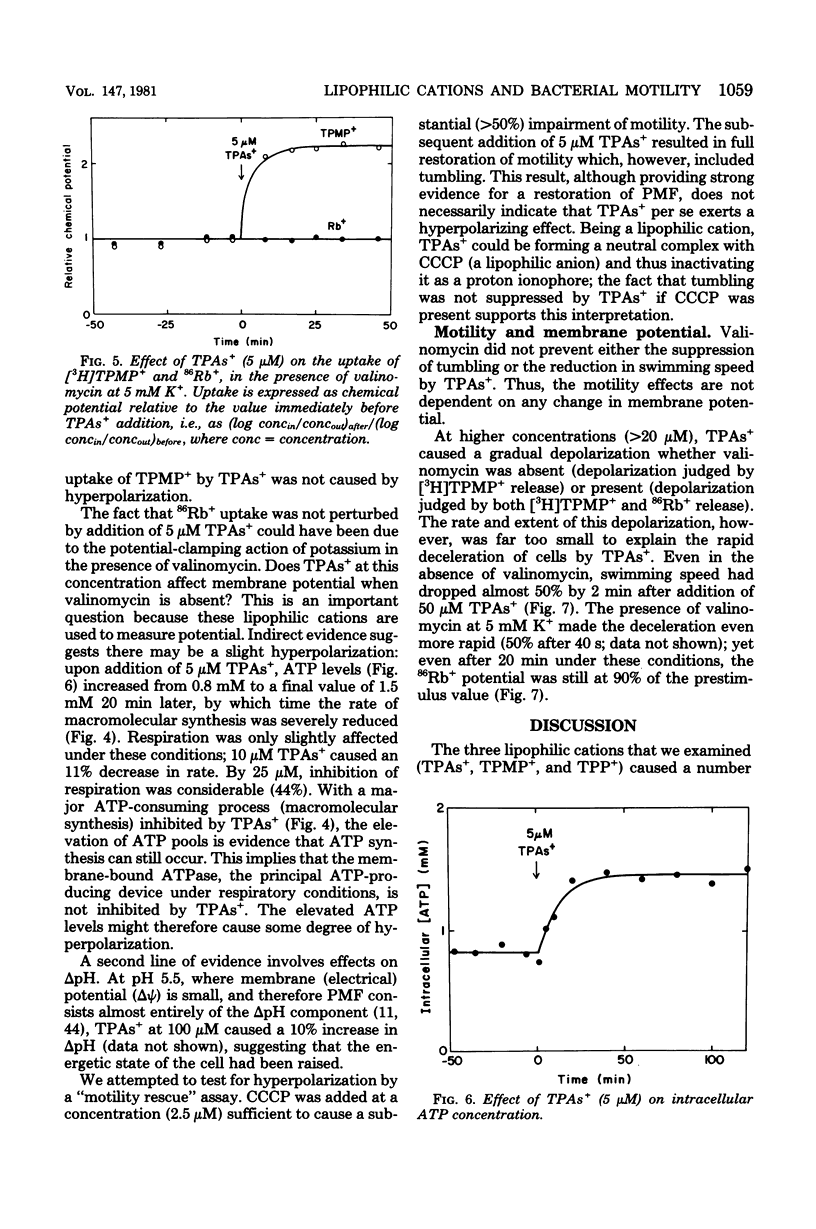
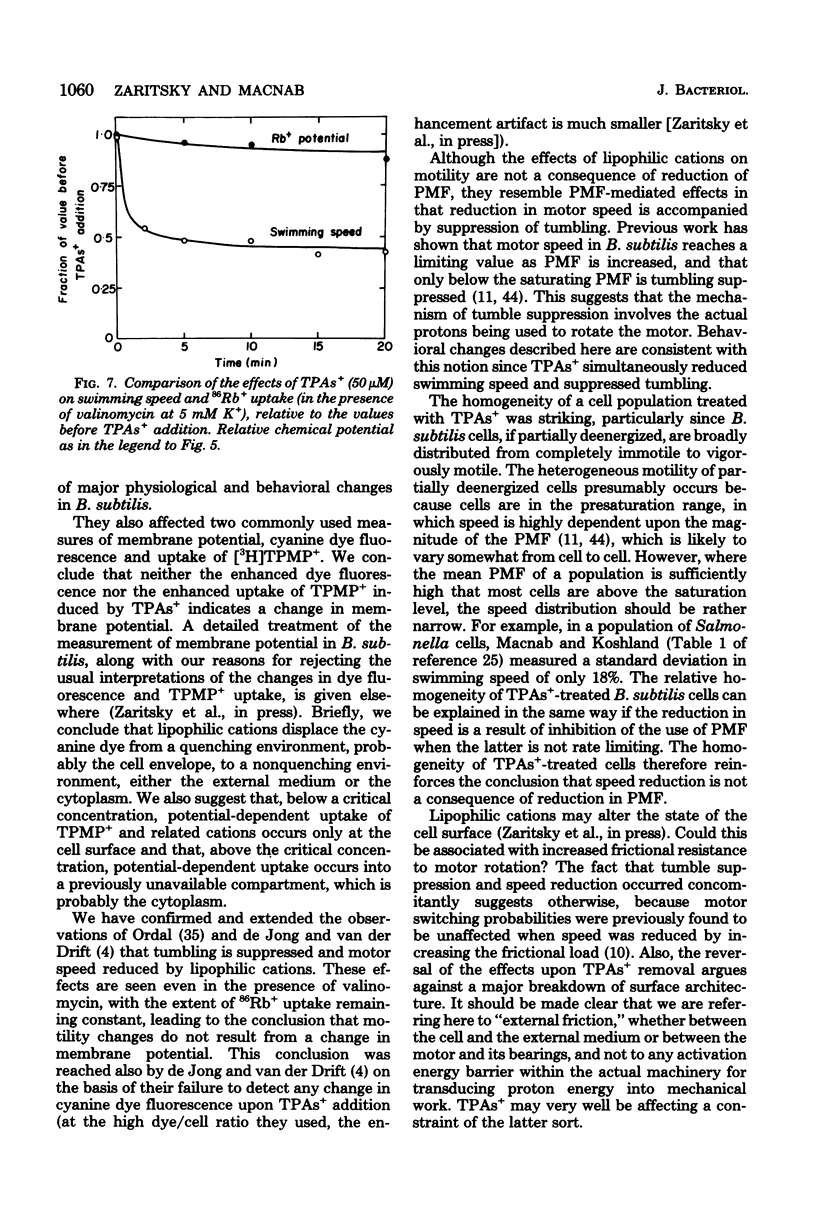
Abstract
Lipophilic cations (tetraphenylarsonium, tetraphenylphosphonium, and triphenylmethylphosphonium) caused a number of major changes in the physiology of Bacillus subtilis. Macromolecular synthesis was inhibited, adenosine 5'-triphosphate concentration increased, swimming speed was reduced, tumbling was suppressed, and the capacity to take up the cations was greatly enhanced; respiration was not significantly altered. The effects occurred at lipophilic cation concentrations in the range commonly employed for measurement of membrane potential. Neither the enhancement of cation uptake nor the motility inhibition was a consequence of alteration of membrane potential, since both effects were still seen in the presence of valinomycin, with the extent of 86Rb+ uptake indicating a constant potential. Because suppression of tumbling accompanied speed reduction, as has also been found when protonmotive force is reduced, it is likely that lipophilic cations are perturbing the process of conversion of proton energy into work, rather than simply causing structural damage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Dynamic properties of bacterial flagellar motors. Nature. 1974 May 3;249(452):77–79. doi: 10.1038/249077a0. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Fein J. E. Helical growth and macrofiber formation of Bacillus subtilis 168 autolytic enzyme deficient mutants. Can J Microbiol. 1980 Mar;26(3):330–337. doi: 10.1139/m80-054. [DOI] [PubMed] [Google Scholar]
- Fein J. E. Possible involvement of bacterial autolytic enzymes in flagellar morphogenesis. J Bacteriol. 1979 Feb;137(2):933–946. doi: 10.1128/jb.137.2.933-946.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J. C., Laris P. C. Electrophysiology of cells and organelles: studies with optical potentiometric indicators. Int Rev Cytol Suppl. 1981;12:177–246. doi: 10.1016/b978-0-12-364373-5.50015-9. [DOI] [PubMed] [Google Scholar]
- Glagolev A. N., Skulachev V. P. The proton pump is a molecular engine of motile bacteria. Nature. 1978 Mar 16;272(5650):280–282. doi: 10.1038/272280a0. [DOI] [PubMed] [Google Scholar]
- Grinius L. L., Jasaitis A. A., Kadziauskas Y. P., Liberman E. A., Skulachev V. P., Topali V. P., Tsofina L. M., Vladimirova M. A. Conversion of biomembrane-produced energy into electric form. I. Submitochondrial particles. Biochim Biophys Acta. 1970 Aug 4;216(1):1–12. doi: 10.1016/0005-2728(70)90153-2. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M., DeFranco A. L., Koshland D. E., Jr Inversion of a behavioral response in bacterial chemotaxis: explanation at the molecular level. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4150–4154. doi: 10.1073/pnas.75.9.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980 Apr 15;138(3):599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. The steady-state counterclockwise/clockwise ratio of bacterial flagellar motors is regulated by protonmotive force. J Mol Biol. 1980 Apr 15;138(3):563–597. doi: 10.1016/s0022-2836(80)80018-0. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Laszlo D. J., Taylor B. L. Aerotaxis in Salmonella typhimurium: role of electron transport. J Bacteriol. 1981 Feb;145(2):990–1001. doi: 10.1128/jb.145.2.990-1001.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Ion transport and rotation of bacterial flagella. Nature. 1977 Jul 28;268(5618):360–362. doi: 10.1038/268360a0. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Bacterial flagella rotating in bundles: a study in helical geometry. Proc Natl Acad Sci U S A. 1977 Jan;74(1):221–225. doi: 10.1073/pnas.74.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M. Bacterial motility and chemotaxis: the molecular biology of a behavioral system. CRC Crit Rev Biochem. 1978;5(4):291–341. doi: 10.3109/10409237809177145. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P. M., Berg H. C. Energetics of flagellar rotation in bacteria. J Mol Biol. 1980 Apr 15;138(3):541–561. doi: 10.1016/s0022-2836(80)80017-9. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S., Shioi J. I., Imae Y., Iida S. Characterization of the Bacillus subtilis motile system driven by an artificially created proton motive force. J Bacteriol. 1979 Oct;140(1):28–36. doi: 10.1128/jb.140.1.28-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Helical Bacillus subtilis macrofibers: morphogenesis of a bacterial multicellular macroorganism. Proc Natl Acad Sci U S A. 1978 May;75(5):2478–2482. doi: 10.1073/pnas.75.5.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Helical growth of Bacillus subtilis: a new model of cell growth. Proc Natl Acad Sci U S A. 1976 May;73(5):1740–1744. doi: 10.1073/pnas.73.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Sensory electrophysiology of bacteria: relationship of the membrane potential to motility and chemotaxis in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4752–4756. doi: 10.1073/pnas.74.11.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W. Calcium ion regulates chemotactic behaviour in bacteria. Nature. 1977 Nov 3;270(5632):66–67. doi: 10.1038/270066a0. [DOI] [PubMed] [Google Scholar]
- Ordal G. W. Control of tumbling in bacterial chemotaxis by divalent cation. J Bacteriol. 1976 May;126(2):706–711. doi: 10.1128/jb.126.2.706-711.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Gibson K. J. Chemotaxis toward amino acids by Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):151–155. doi: 10.1128/jb.129.1.151-155.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotaxis away from uncouplers of oxidative phosphorylation in Bacillus subtilis. Science. 1975 Sep 5;189(4205):802–805. doi: 10.1126/science.808854. [DOI] [PubMed] [Google Scholar]
- Ordal G. W. Recognition sites for chemotactic repellents of Bacillus subtilis. J Bacteriol. 1976 Apr;126(1):72–79. doi: 10.1128/jb.126.1.72-79.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Ridgway H. G., Silverman M., Simon M. I. Localization of proteins controlling motility and chemotaxis in Escherichia coli. J Bacteriol. 1977 Nov;132(2):657–665. doi: 10.1128/jb.132.2.657-665.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Shioi J. I., Imae Y., Oosawa F. Protonmotive force and motility of Bacillus subtilis. J Bacteriol. 1978 Mar;133(3):1083–1088. doi: 10.1128/jb.133.3.1083-1088.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi J. I., Matsuura S., Imae Y. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):891–897. doi: 10.1128/jb.144.3.891-897.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S. The use of cyanine dyes for the determination of membrane potentials in cells, organelles, and vesicles. Methods Enzymol. 1979;55:689–695. doi: 10.1016/0076-6879(79)55077-0. [DOI] [PubMed] [Google Scholar]
- de Jong M. H., van der Drift C. Control of the chemotactic behavior of Bacillus subtilis cells. Arch Microbiol. 1978 Jan 23;116(1):1–8. doi: 10.1007/BF00408727. [DOI] [PubMed] [Google Scholar]