Abstract
Cell-free extracts, membranous fractions, and cell wall preparations from Schizosaccharomyces pombe were examined for the presence of (1 → 3)-β-, (1 → 3)-α-, and (1 → 6)-β-glucanase activities. The various glucanases were assayed in cells at different growth stages. Only (1 → 3)-β-glucanase activity was found, and this was associated with the cell wall fraction. Chromatographic fractionation of the crude enzyme revealed two endo-(1 → 3)-β-glucanases, designated as glucanase I and glucanase II. Glucanase I consisted of two subunits of molecular weights 78,500 and 82,000, and glucanase II was a single polypeptide of 75,000. Although both enzymes had similar substrate specificities and similar hydrolytic action on laminarin, glucanase II had much higher hydrolytic activity on isolated cell walls of S. pombe. On the basis of differential lytic activity on cell walls, glucanase II was shown to be present in conjugating cells and highest in sporulating cells. Glucanase II appeared to be specifically involved in conjugation and sporulation since vegetative cells and nonconjugating and nonsporulating cells did not contain this enzyme. The appearance of glucanase II in conjugating cells may be due to de novo enzyme synthesis since no activation could be demonstrated by combining extracts from vegetative and conjugating cells. Increased glucanase activity occurred when walls from conjugating cells were combined with walls from sporulating cells. Studies with trypsin and proteolytic inhibitors suggest that glucanase II exists as a zymogen in conjugating cells. A temperature-sensitive mutant of S. pombe was isolated which lysed at 37°C. Glucanase activity was higher in vegetative cells held at 37°C than cells held at 25°C. Unlike the wild-type strain, this mutant contained glucanase II activity during vegetative growth and may be a regulatory mutant.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N. The structure of the yeast cell wall. Solubilization of a marker enzyme, -fructofuranosidase, by the autolytic enzyme system. J Biol Chem. 1972 Feb 25;247(4):1161–1169. [PubMed] [Google Scholar]
- Barras D. R. A -glucan endo-hydrolase from Schizosaccharomyces pombe and its role in cell wall growth. Antonie Van Leeuwenhoek. 1972;38(1):65–80. doi: 10.1007/BF02328078. [DOI] [PubMed] [Google Scholar]
- Bush D. A., Horisberger M., Horman I., Wursch P. The wall structure of Schizosaccharomyces pombe. J Gen Microbiol. 1974 Mar;81(1):199–206. doi: 10.1099/00221287-81-1-199. [DOI] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. A molecular model for morphogenesis: the primary septum of yeast. Curr Top Cell Regul. 1974;8(0):1–32. doi: 10.1016/b978-0-12-152808-9.50008-0. [DOI] [PubMed] [Google Scholar]
- Crandall M., Egel R., Mackay V. L. Physiology of mating in three yeasts. Adv Microb Physiol. 1977;15:307–398. doi: 10.1016/s0065-2911(08)60319-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Farkas V., Biely P., Bauer S. Extracellular beta-glucanases of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Sep 15;321(1):246–255. doi: 10.1016/0005-2744(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Manners D. J. Isolation and composition of an alkali-soluble glucan from the cell walls of Saccharomyces cerevisiae. J Gen Microbiol. 1976 May;94(1):180–192. doi: 10.1099/00221287-94-1-180. [DOI] [PubMed] [Google Scholar]
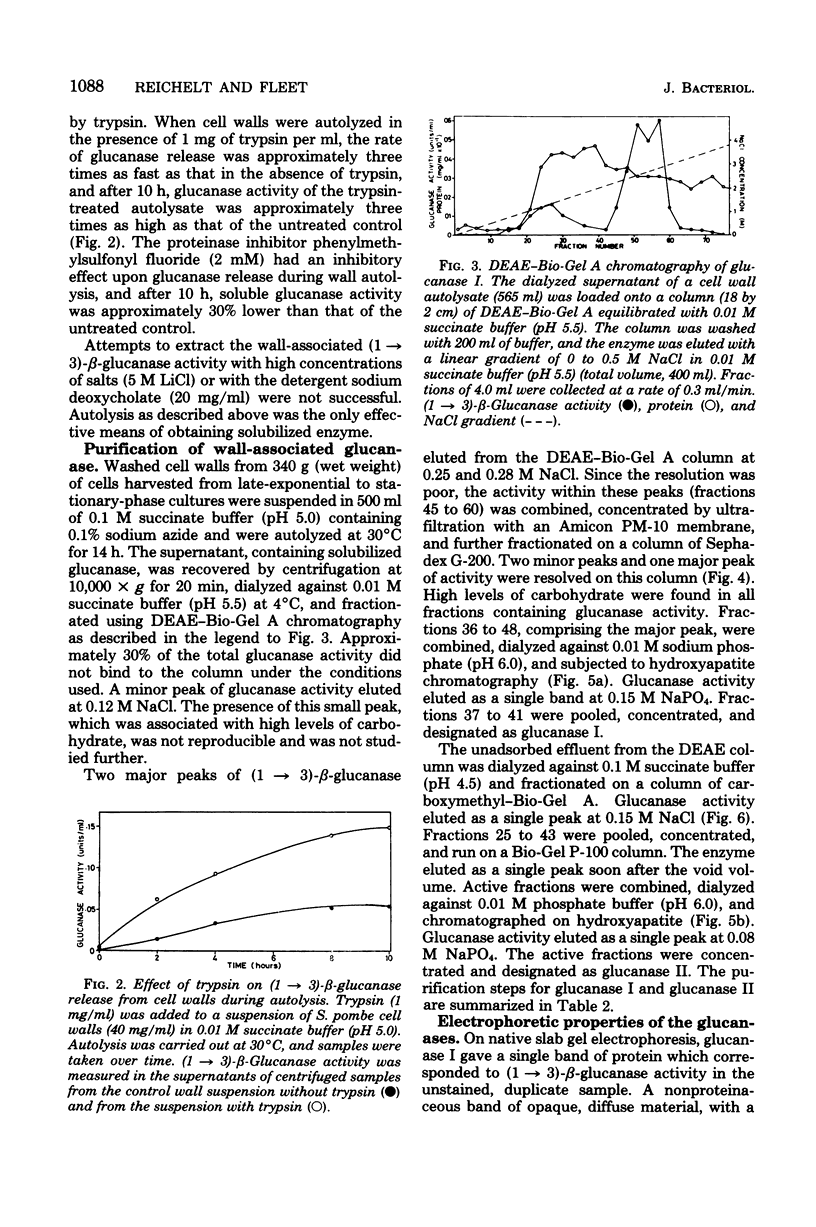
- Fleet G. H., Phaff H. J. Glucanases in Schizosaccharomyces. Isolation and properties of the cell wall-associated beta(1 leads to 3)-glucanases. J Biol Chem. 1974 Mar 25;249(6):1717–1728. [PubMed] [Google Scholar]
- Kritzman G., Chet I., Henis Y. Localization of beta-(1,3)-glucanase in the mycelium of Sclerotium rolfsii. J Bacteriol. 1978 May;134(2):470–475. doi: 10.1128/jb.134.2.470-475.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
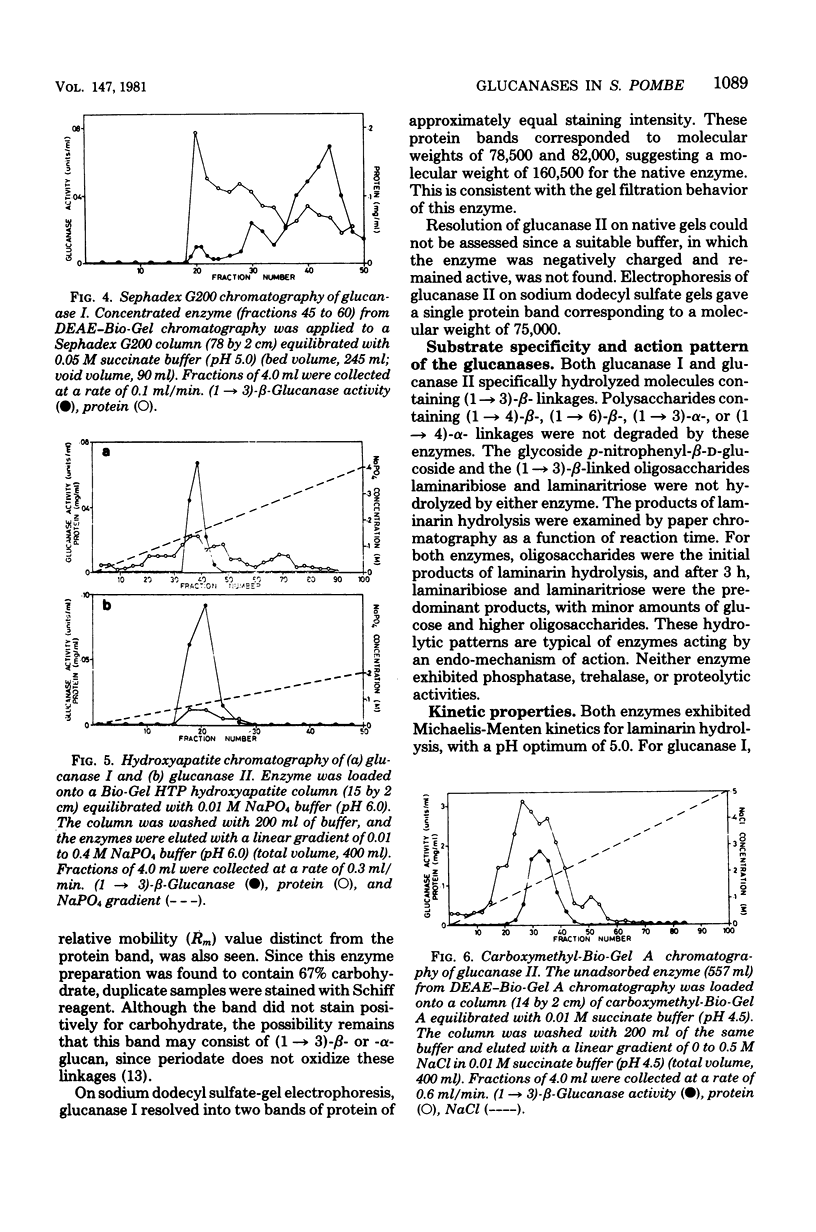
- Kröning A., Egel R. Autolytic activities associated with conjugation and sporulation in fission yeast. Arch Microbiol. 1974;99(3):241–249. doi: 10.1007/BF00696238. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Dalbec J. M. Purification and properties of two proteinases from Saccharomyces cerevisiae. Arch Biochem Biophys. 1967 Apr;120(1):42–48. doi: 10.1016/0003-9861(67)90595-4. [DOI] [PubMed] [Google Scholar]
- Manners D. J., Masson A. J., Patterson J. C. The heterogeneity of glucan preparations from the walls of various yeasts. J Gen Microbiol. 1974 Feb;80(2):411–417. doi: 10.1099/00221287-80-2-411. [DOI] [PubMed] [Google Scholar]
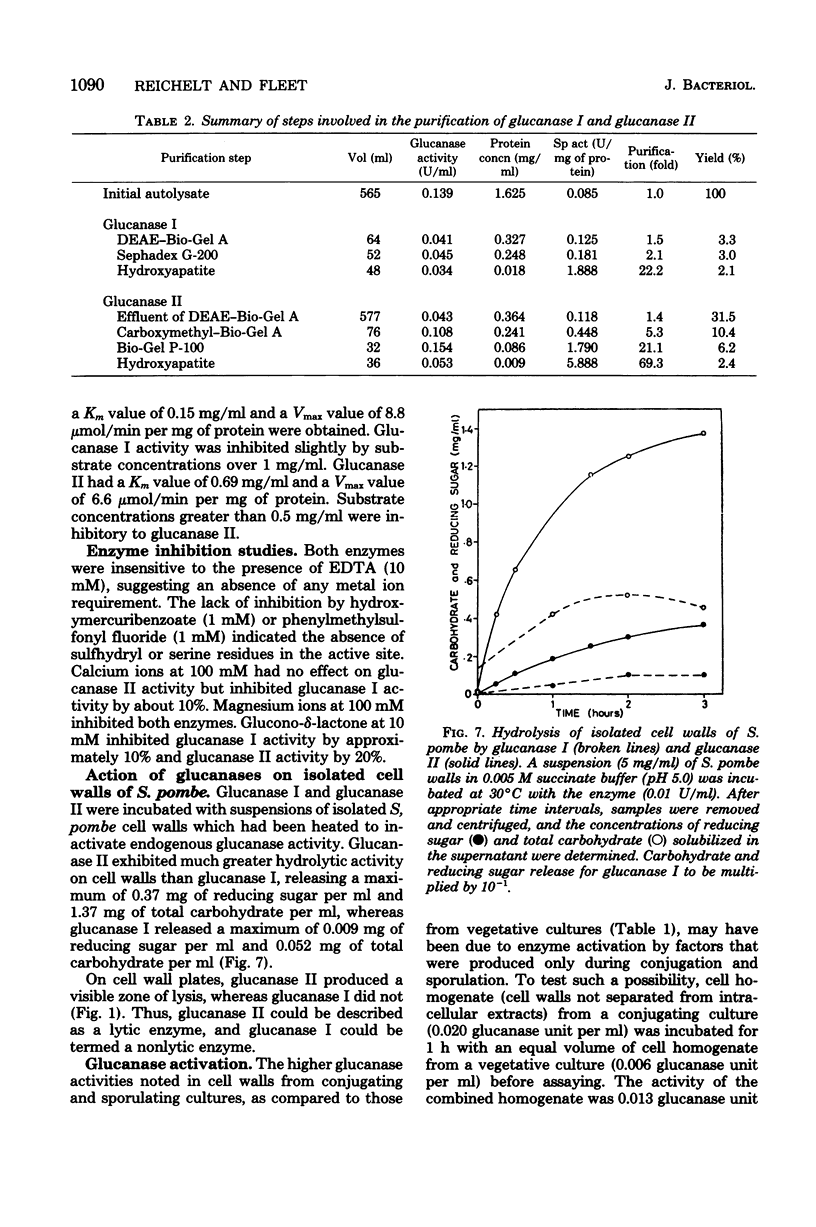
- Manners D. J., Masson A. J., Patterson J. C. The structure of a beta-(1 leads to 3)-D-glucan from yeast cell walls. Biochem J. 1973 Sep;135(1):19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. T., Phaff H. J. Survey for alpha-(1 leads to 3)-glucanase activity among yeasts. J Bacteriol. 1977 Aug;131(2):702–706. doi: 10.1128/jb.131.2.702-706.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notario V., Villa T. G., Benítez T., Villanueva J. R. Beta-glucanases in the yeast Cryptococcus albidus var. aerius. Production and separation of beta-glucanases in asynchronous cultures. Can J Microbiol. 1976 Feb;22(2):261–268. doi: 10.1139/m76-035. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Rosenberger R. F. Distribution of autolysins in hyphae of Aspergillus nidulans: evidence for a lipid-mediated attachment to hyphal walls. J Bacteriol. 1978 Sep;135(3):741–747. doi: 10.1128/jb.135.3.741-747.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Biogenesis of the wall in bacterial morphogenesis. Adv Microb Physiol. 1979;19:1–62. doi: 10.1016/s0065-2911(08)60197-6. [DOI] [PubMed] [Google Scholar]
- Santos T., Sánchez M., Villanueva J. R., Nombela C. Derepression of beta-1,3-glucanases in Penicillium italicum: localization of the various enzymes and correlation with cell wall glucan mobilization and autolysis. J Bacteriol. 1979 Jan;137(1):6–12. doi: 10.1128/jb.137.1.6-12.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA H., PHAFF H. J. ENZYMATIC HYDROLYSIS OF YEAST CELL WALLS. I. ISOLATION OF WALL-DECOMPOSING ORGANISMS AND SEPARATION AND PURIFICATION OF LYTIC ENZYMES. J Bacteriol. 1965 Jun;89:1570–1580. doi: 10.1128/jb.89.6.1570-1580.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa T. G., Notario V., Villanueva J. R. Beta-glucanases of the yeast Pichia polymorpha. Arch Microbiol. 1975 Jun 22;104(2):201–206. doi: 10.1007/BF00447325. [DOI] [PubMed] [Google Scholar]
- Villa T. G., Notario V., Villanueva J. R. Occurrence of an endo-1,3-beta-glucanase in culture fluids of the yeast Candida utilis. Purification and characterization of the enzyme activity. Biochem J. 1979 Jan 1;177(1):107–114. doi: 10.1042/bj1770107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey F., Santos T., García-Acha I., Nombela C. Synthesis of 1,3-beta-glucanases in Saccharomyces cerevisiae during the mitotic cycle, mating, and sporulation. J Bacteriol. 1979 Sep;139(3):924–931. doi: 10.1128/jb.139.3.924-931.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]





