Abstract
A number of mutant alleles affecting the Pst phosphate transport system have been divided into three complementation groups on the basis of constitutive alkaline phosphatase activity in appropriate partial diploid strains. The three complementation groups were represented by the alleles pstA2 and phoT32 and the newly described allele pstB401. The two alleles phoS28 and phoS21 appeared to be polar. The phoS28 allele affected both the phoT and pstB genes but not the pstA gene, whereas the phoS21 allele appeared to be a mutation in the pstA gene exerting polar effects on both the pstB and phoT genes. It was concluded that the three genes pstA, pstB, and phoT were part of an operon and that the phosphate-binding protein was not coded for by any of these genes. The phoS gene, defined as the structural gene for the phosphate-binding protein, is also part of the operon, but the phoS28 and phoS21 alleles are not mutations in the phoS gene and were reclassified as pho-28 and pho-21 alleles. The gene order was concluded to be pstA-(pstB-phoT)-phoS, with the pstA gene promotor proximal and the direction of transcription opposite to that of the nearby unc operon.
Full text
PDF


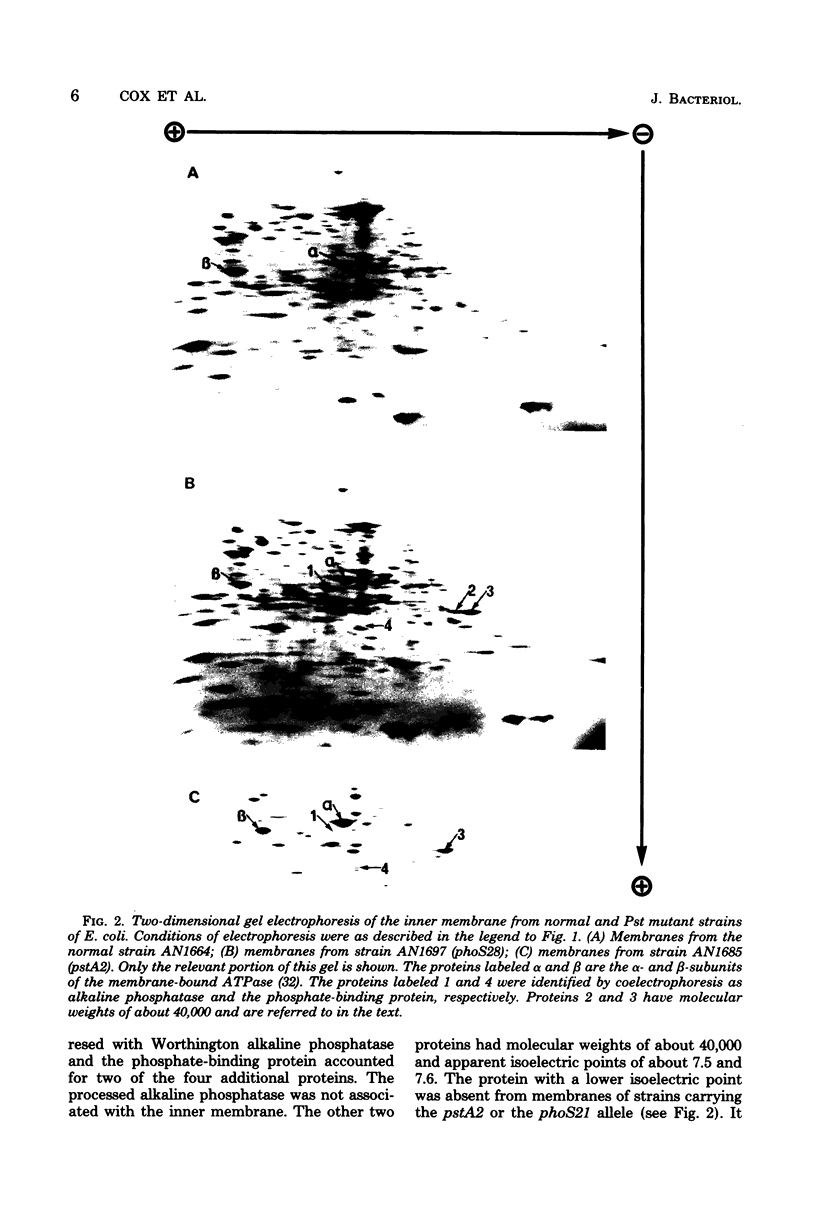



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono H., Otsuji N. Genetic mapping of regulator gene phoS for alkaline phosphatase in Escherichia coli. J Bacteriol. 1968 Mar;95(3):1182–1183. doi: 10.1128/jb.95.3.1182-1183.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast M., Boos W. Co-regulation in Escherichia coli of a novel transport system for sn-glycerol-3-phosphate and outer membrane protein Ic (e, E) with alkaline phosphatase and phosphate-binding protein. J Bacteriol. 1980 Jul;143(1):142–150. doi: 10.1128/jb.143.1.142-150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast M., Boos W. Purification and properties of the sn-glycerol 3-phosphate-binding protein of Escherichia coli. J Biol Chem. 1979 Nov 10;254(21):10931–10935. [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Inouye H., Model P., Beckwith J. Processing of alkaline phosphatase precursor to the mature enzyme by an Escherichia coli inner membrane preparation. J Bacteriol. 1980 May;142(2):726–728. doi: 10.1128/jb.142.2.726-728.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A. Isolation and characterization of mutants of Escherichia coli K-12 affected in oxidative phosphorylation of quinone biosynthesis. Methods Enzymol. 1979;56:106–117. doi: 10.1016/0076-6879(79)56013-3. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Cox G. B., Langman L., Ash G., Becker M., Gibson F. Three genes coding for subunits of the membrane sector (F0) of the Escherichia coli adenosine triphosphatase complex. J Bacteriol. 1981 Jan;145(1):200–210. doi: 10.1128/jb.145.1.200-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Langman L., Cox G. B., Yanofsky C., Gibson F. Subunits of the adenosine triphosphatase complex translated in vitro from the Escherichia coli unc operon. J Bacteriol. 1980 Jul;143(1):8–17. doi: 10.1128/jb.143.1.8-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- GAREN A., OTSUJI N. ISOLATION OF A PROTEIN SPECIFIED BY A REGULATOR GENE. J Mol Biol. 1964 Jun;8:841–852. doi: 10.1016/s0022-2836(64)80165-0. [DOI] [PubMed] [Google Scholar]
- Gerdes R. G., Rosenberg H. The relationship between the phosphate-binding protein and a regulator gene product from Escherichia coli. Biochim Biophys Acta. 1974 May 10;351(1):77–86. doi: 10.1016/0005-2795(74)90066-x. [DOI] [PubMed] [Google Scholar]
- Gerdes R. G., Strickland K. P., Rosenberg H. Restoration of phosphate transport by the phosphate-binding protein in spheroplasts of Escherichia coli. J Bacteriol. 1977 Aug;131(2):512–518. doi: 10.1128/jb.131.2.512-518.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. Partial diploids of Escherichia coli carrying normal and mutant alleles affecting oxidative phosphorylation. Biochem J. 1977 Mar 15;162(3):665–670. doi: 10.1042/bj1620665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Downie J. A., Cox G. B., Radik J. Mu-induced polarity in the unc operon of Escherichia coli. J Bacteriol. 1978 Jun;134(3):728–736. doi: 10.1128/jb.134.3.728-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. A possible negative feedback phenomenon controlling formation of alkaline phosphomonoesterase in Escherichia coli. Nature. 1959 May 30;183(4674):1529–1530. doi: 10.1038/1831529b0. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz R., Bittan R., Yagil E. Complementation tests between alkaline phosphatase-constitutive mutants (phoS and phoT) of Escherichia coli. J Bacteriol. 1981 Mar;145(3):1432–1435. doi: 10.1128/jb.145.3.1432-1435.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H., Schlesinger M. J., Bracha M., Yagil E. Pleiotropic effects of mutations involved in the regulation of Escherichia coli K-12 alkaline phosphatase. J Bacteriol. 1974 Aug;119(2):583–592. doi: 10.1128/jb.119.2.583-592.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Harold F. M. Energy coupling to the transport of inorganic phosphate in Escherichia coli K12. Biochem J. 1979 Jan 15;178(1):133–137. doi: 10.1042/bj1780133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H. Transport of iron into bacterial cells. Methods Enzymol. 1979;56:388–394. doi: 10.1016/0076-6879(79)56036-4. [DOI] [PubMed] [Google Scholar]
- Russell L. M., Rosenberg H. Linked transport of phosphate, potassium ions and protons in Escherichia coli. Biochem J. 1979 Oct 15;184(1):13–21. doi: 10.1042/bj1840013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E., Brown K., Yanofsky C. Mitomycin C-induced expression of trpA of Salmonella typhimurium inserted into the plasmid ColE1. J Bacteriol. 1977 Jan;129(1):388–394. doi: 10.1128/jb.129.1.388-394.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Fayle D. R., Downie J. A., Gibson F., Cox G. B. Properties of membranes from mutant strains of Escherichia coli in which the beta-subunit of the adenosine triphosphatase is abnormal. Biochem J. 1979 Apr 15;180(1):111–118. doi: 10.1042/bj1800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Shimizu M., Saito H., Ikeda Y. Studies of viable T4 bacteriophage containing cytosine-substituted DNA (T4dC phage). II. Cleavage of T4dC DNA by endonuclease SalI and bam HI. Mol Gen Genet. 1979 Jan 5;168(1):49–53. doi: 10.1007/BF00267932. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Silberstein N., Gerdes R. G. Co-regulation of the phosphate-binding protein and alkaline phosphatase synthesis in Escherichia coli. J Bacteriol. 1976 Jul;127(1):656–659. doi: 10.1128/jb.127.1.656-659.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckier G., Torriani A. Genetic and physiological tests of three phosphate-specific transport mutants of Escherichia coli. J Bacteriol. 1981 Mar;145(3):1249–1256. doi: 10.1128/jb.145.3.1249-1256.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]