Abstract
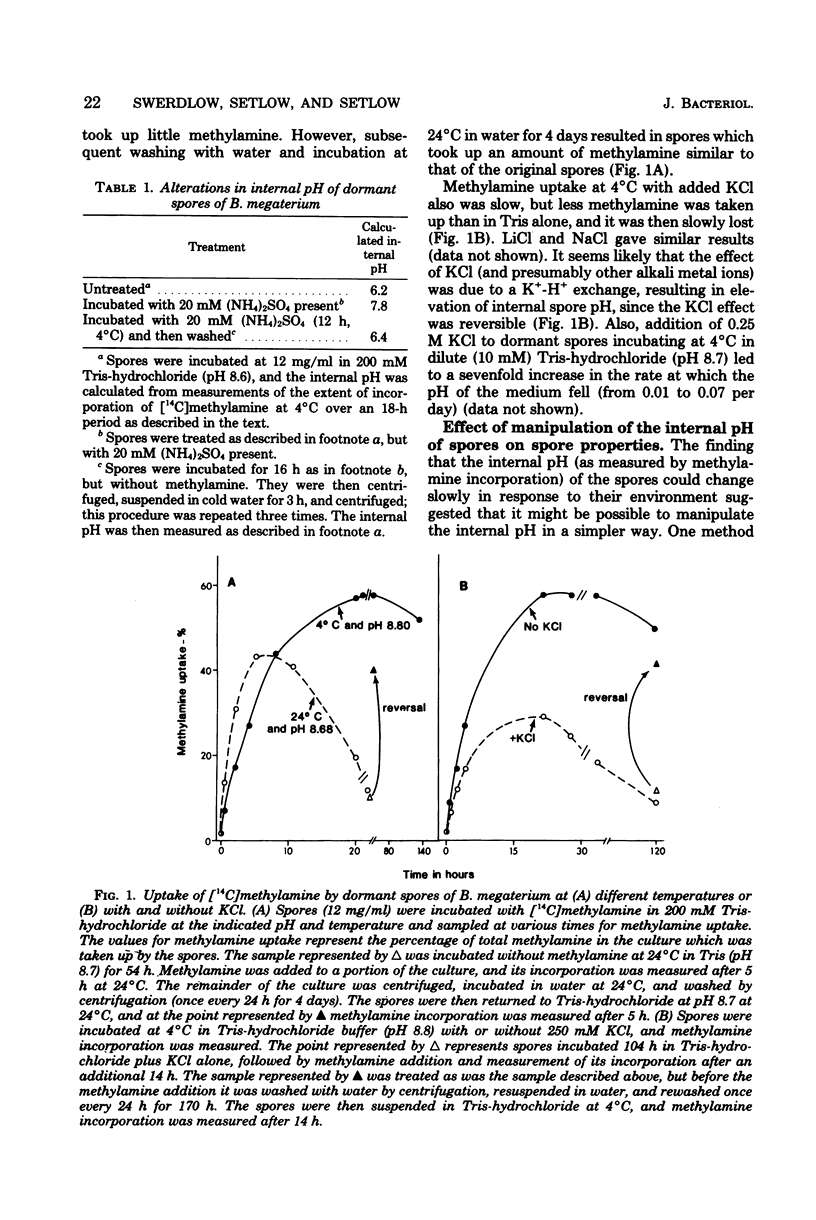
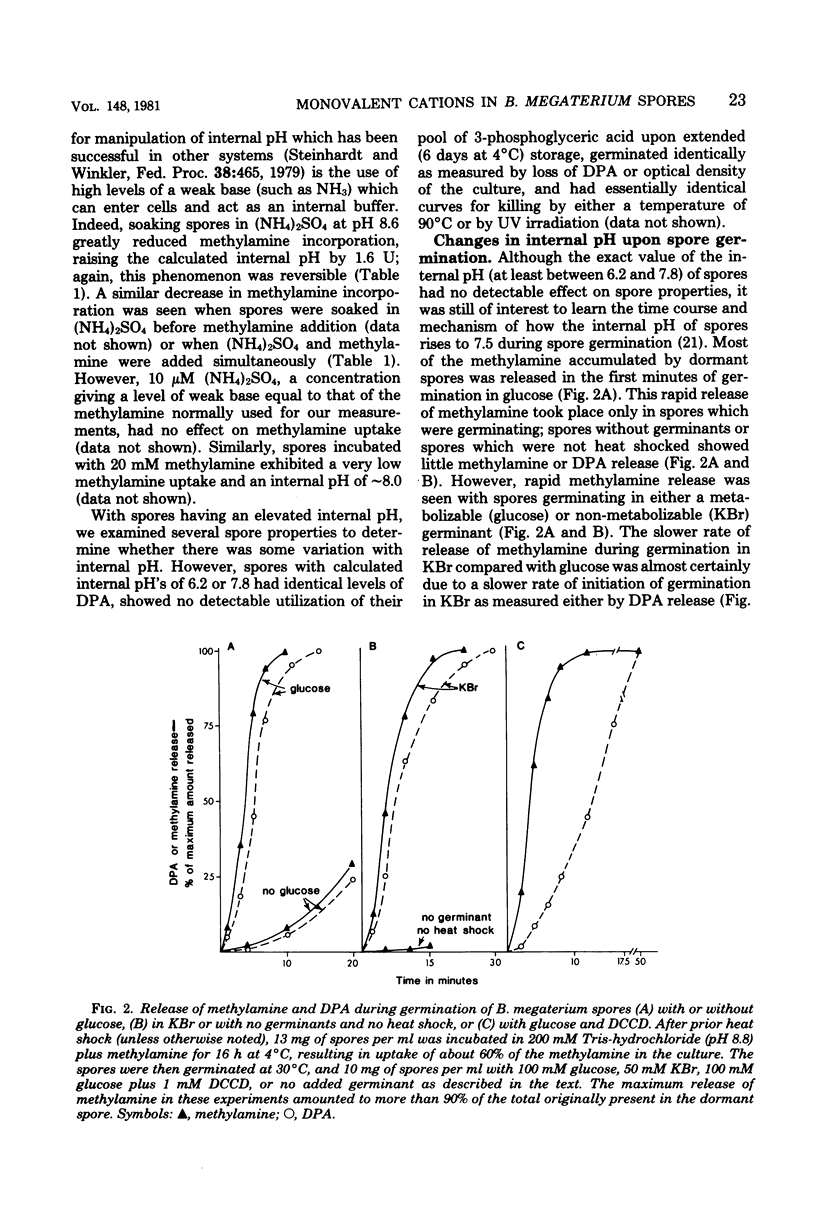
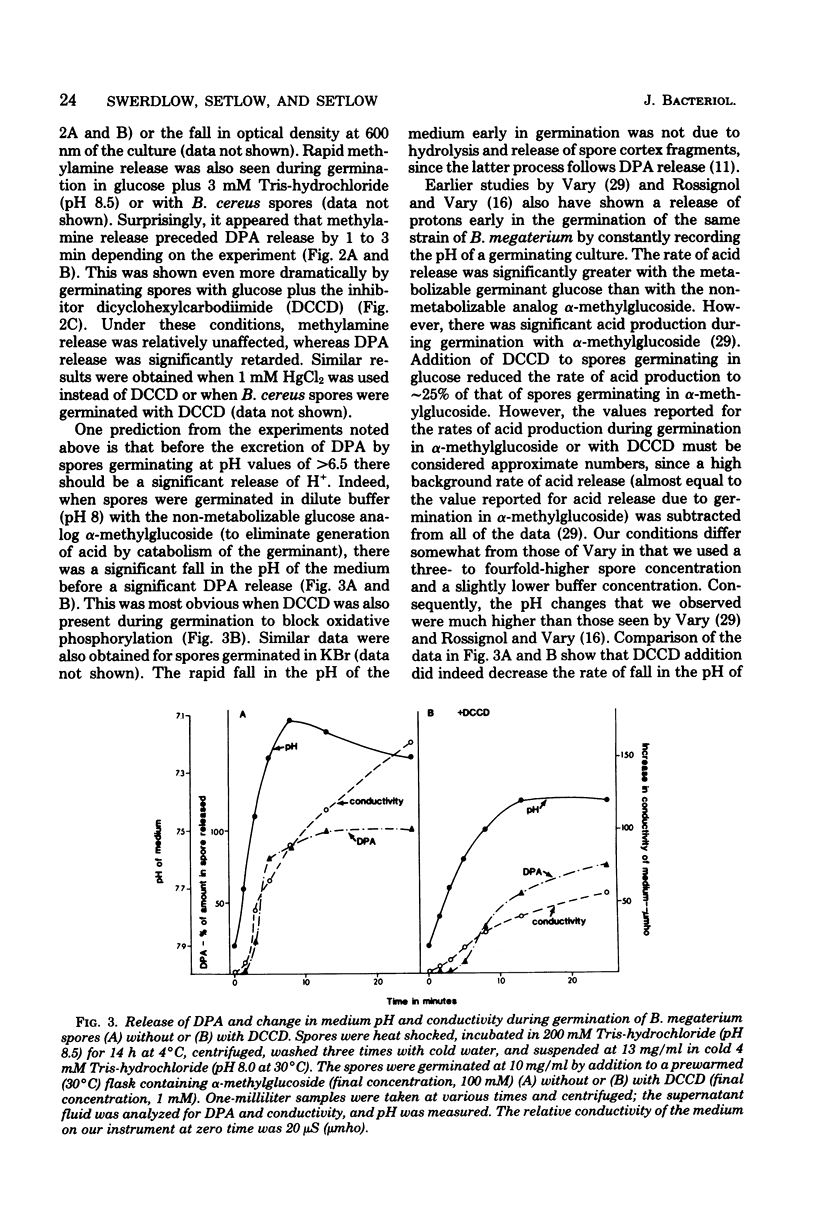
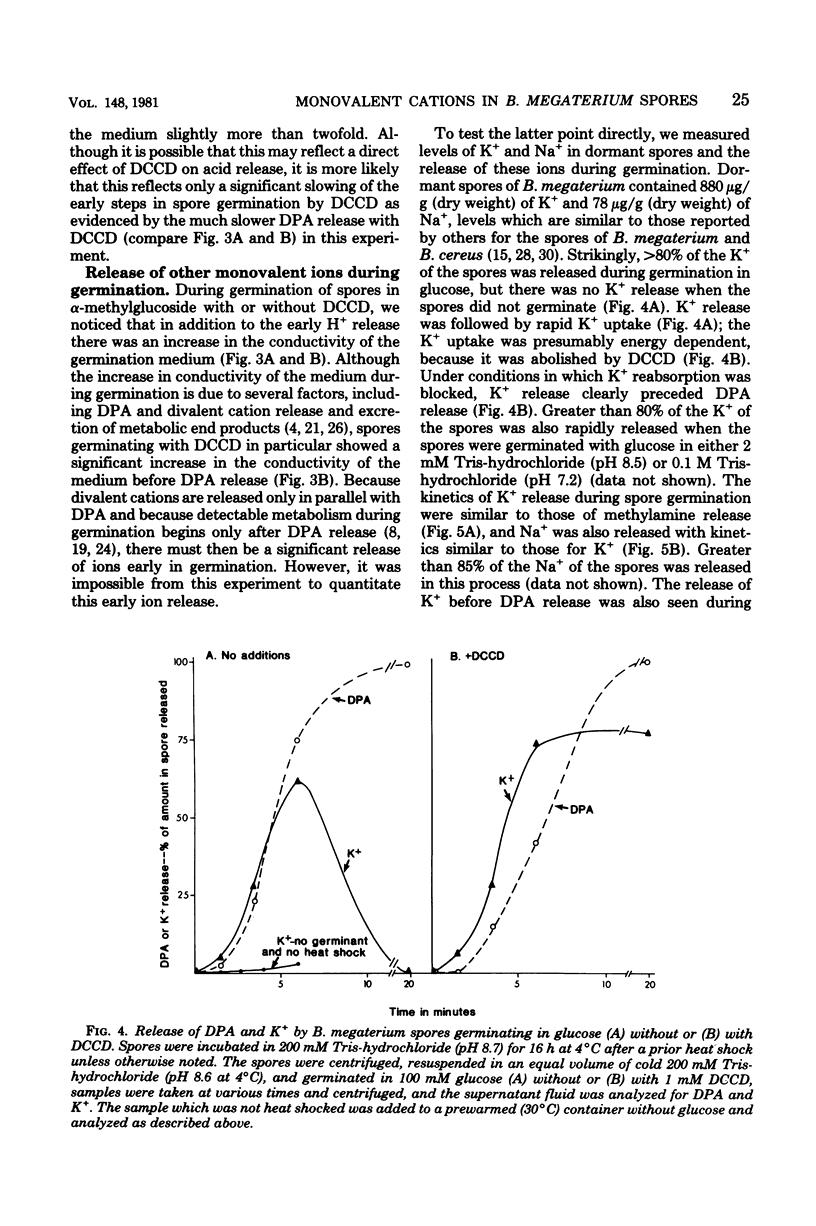
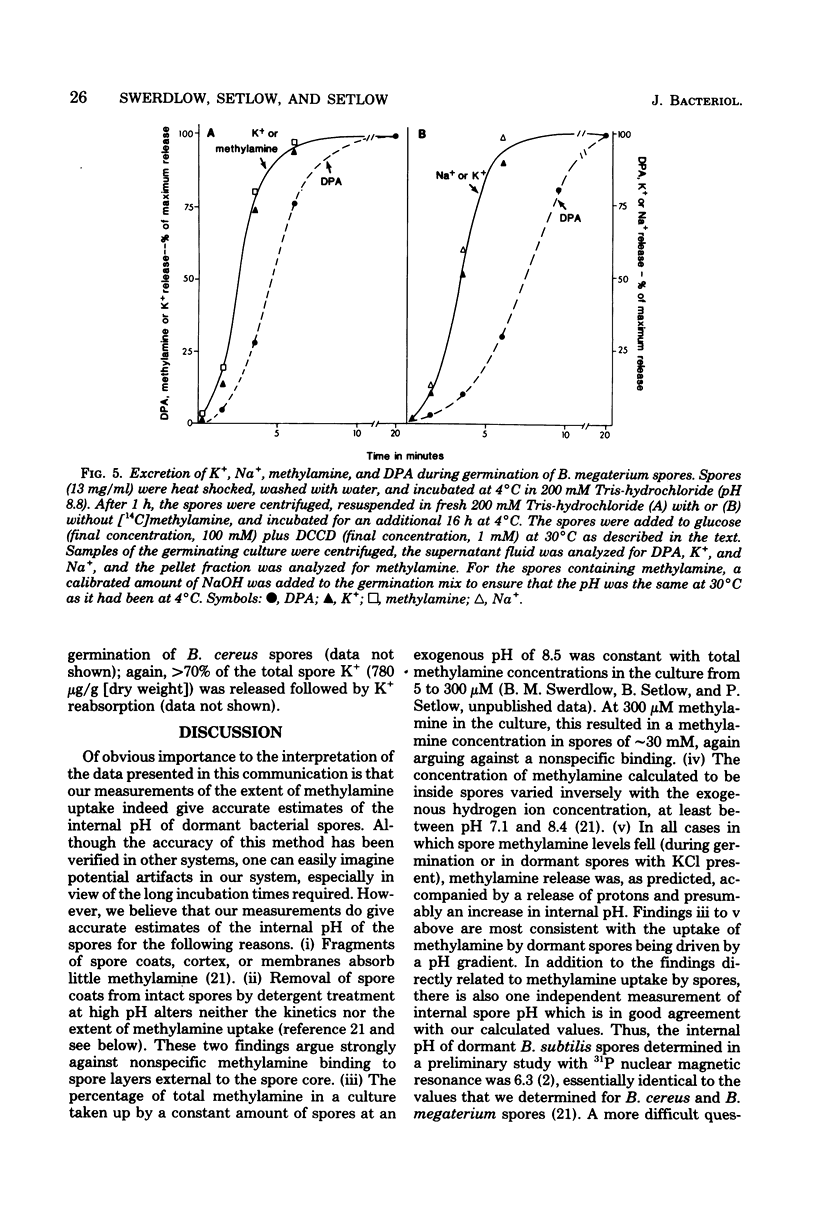
Previous investigators using the extent of uptake of the weak base methylamine to measure internal pH have shown that the pH in the core region of dormant spores of Bacillus megaterium is 6.3 to 6.5. Elevation of the internal pH of spores by 1.6 U had no significant effect on their degree of dormancy or their heat or ultraviolet light resistance. Surprisingly, the rate of methylamine uptake into dormant spores was slow (time for half-maximal uptake, 2.5 h at 24 degrees C). Most of the methylamine taken up by dormant spores was rapidly (time for half-maximal uptake, less than 3 min) released during spore germination as the internal pH of spores rose to approximately 7.5. This rise in internal spore pH took place before dipicolinic acid release, was not abolished by inhibition of energy metabolism, and during germination at pH 8.0 was accompanied by a decrease in the pH of the germination medium. Also accompanying the rise in internal spore pH during germination was the release of greater than 80% of the spores K+ and Na+. The K+ was subsequently reabsorbed in an energy-dependent process. These data indicate (i) that between pH 6.2 and 7.8 internal spore pH has little effect on dormant spore properties, (ii) that there is a strong permeability barrier in dormant spores to movement of charged molecules and small uncharged molecules, and (iii) that extremely early in spore germination this permeability barrier is breached, allowing rapid release of internal monovalent cations (H+, Na+, and K+).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDERTON G., SNELL N. Base exchange and heat resistance in bacterial spores. Biochem Biophys Res Commun. 1963 Jan 31;10:139–143. doi: 10.1016/0006-291x(63)90039-1. [DOI] [PubMed] [Google Scholar]
- Barton J. K., den Hollander J. A., Lee T. M., MacLaughlin A., Shulman R. G. Measurement of the internal pH of yeast spores by 31P nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1980 May;77(5):2470–2473. doi: 10.1073/pnas.77.5.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dring G. J., Gould G. W. Sequence of events during rapid germination of spores of Bacillus cereus. J Gen Microbiol. 1971 Jan;65(1):101–104. doi: 10.1099/00221287-65-1-101. [DOI] [PubMed] [Google Scholar]
- Epel D. The program of fertilization. Sci Am. 1977 Nov;237(5):128–138. doi: 10.1038/scientificamerican1177-128. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Ion permeability of isolated chromaffin granules. J Gen Physiol. 1976 Dec;68(6):601–631. doi: 10.1085/jgp.68.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara T., Ichikawa T., Kondo M. Location of elements in ashed spores of Bacillus megaterium. Microbiol Immunol. 1980;24(6):495–506. doi: 10.1111/j.1348-0421.1980.tb02853.x. [DOI] [PubMed] [Google Scholar]
- Rossignol D. P., Vary J. C. Biochemistry of L-proline-triggered germination of Bacillus megaterium spores. J Bacteriol. 1979 May;138(2):431–441. doi: 10.1128/jb.138.2.431-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman Y., Fields M. L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1968 Jan;22(1):168–168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1977 Feb;129(2):857–865. doi: 10.1128/jb.129.2.857-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Localization of low-molecular-weight basic proteins in Bacillus megaterium spores by cross-linking with ultraviolet light. J Bacteriol. 1979 Aug;139(2):486–494. doi: 10.1128/jb.139.2.486-494.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Shay L. K., Vary J. C., Setlow P. Production of large amounts of acetate during germination of Bacillus megaterium spores in the absence of exogenous carbon sources. J Bacteriol. 1977 Nov;132(2):744–746. doi: 10.1128/jb.132.2.744-746.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow P. Regulation of phosphoglycerate phosphomutase in developing forespores and dormant and germinated spores of Bacillus megaterium by the level of free manganous ions. J Bacteriol. 1979 Sep;139(3):889–898. doi: 10.1128/jb.139.3.889-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. S., Eaton M. W., Johnstone K., Barrett M. D., Ellar D. J. An investigation of membrane fluidity changes during sporulation and germination of Bacillus megaterium K.M. measured by electron spin and nuclear magnetic resonance spectroscopy. Biochim Biophys Acta. 1980 Aug 4;600(2):270–290. doi: 10.1016/0005-2736(80)90432-0. [DOI] [PubMed] [Google Scholar]
- Stewart M., Somlyo A. P., Somlyo A. V., Shuman H., Lindsay J. A., Murrell W. G. Distribution of calcium and other elements in cryosectioned Bacillus cereus T spores, determined by high-resolution scanning electron probe x-ray microanalysis. J Bacteriol. 1980 Jul;143(1):481–491. doi: 10.1128/jb.143.1.481-491.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



