Abstract
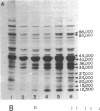
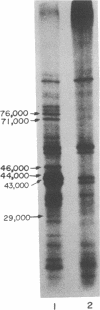
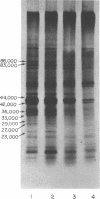
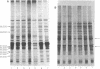
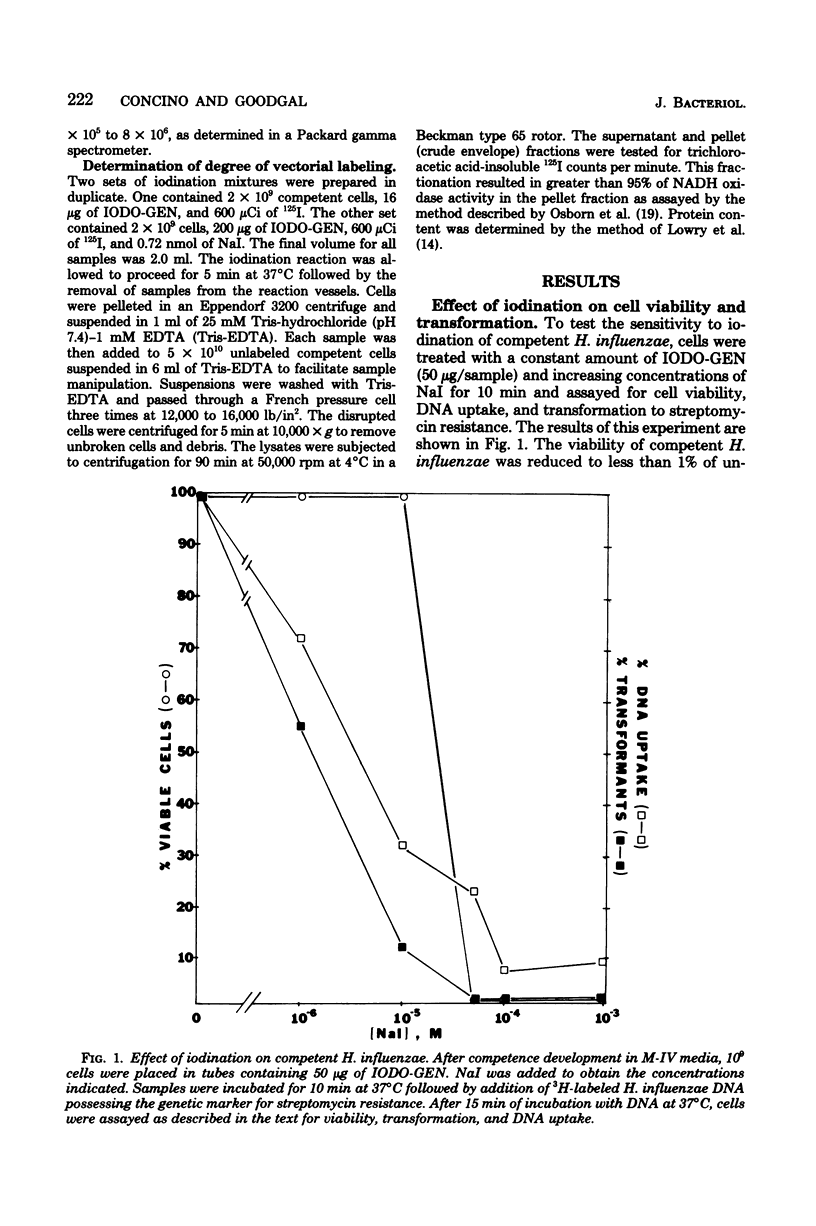
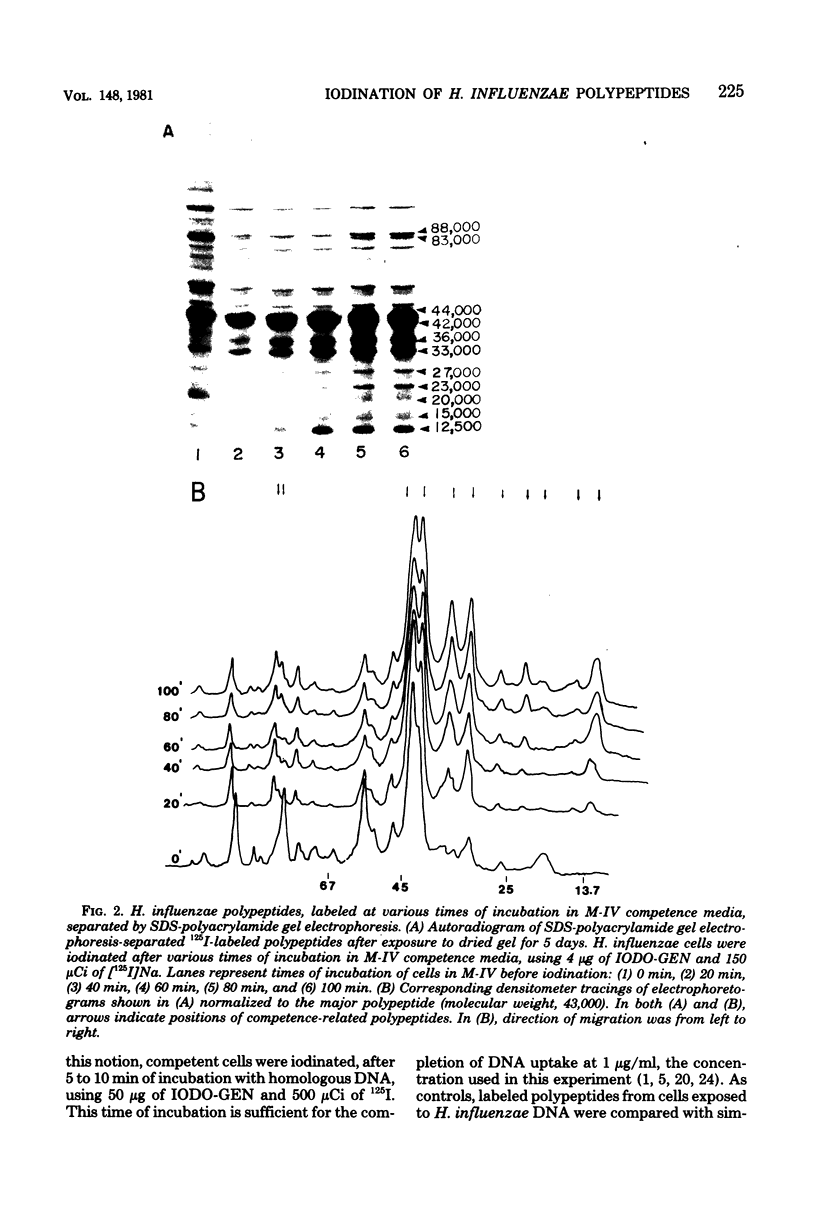
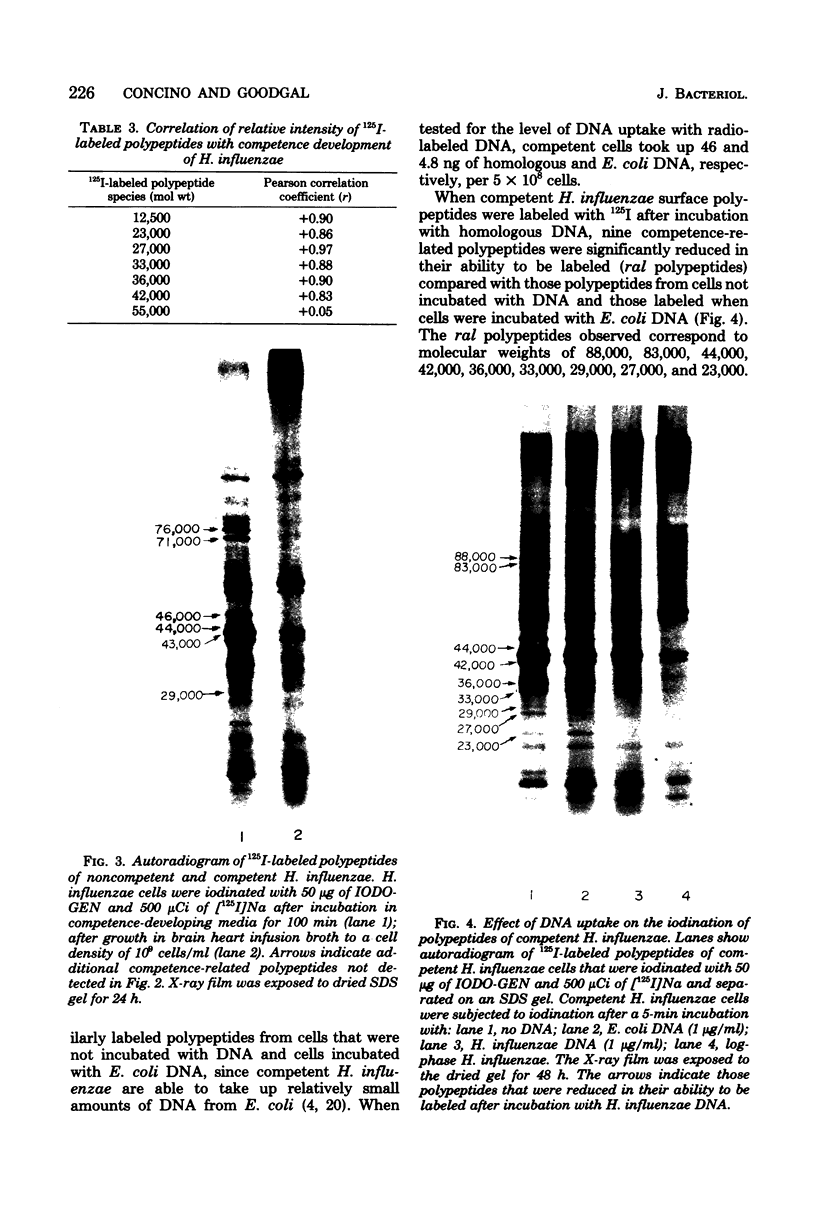
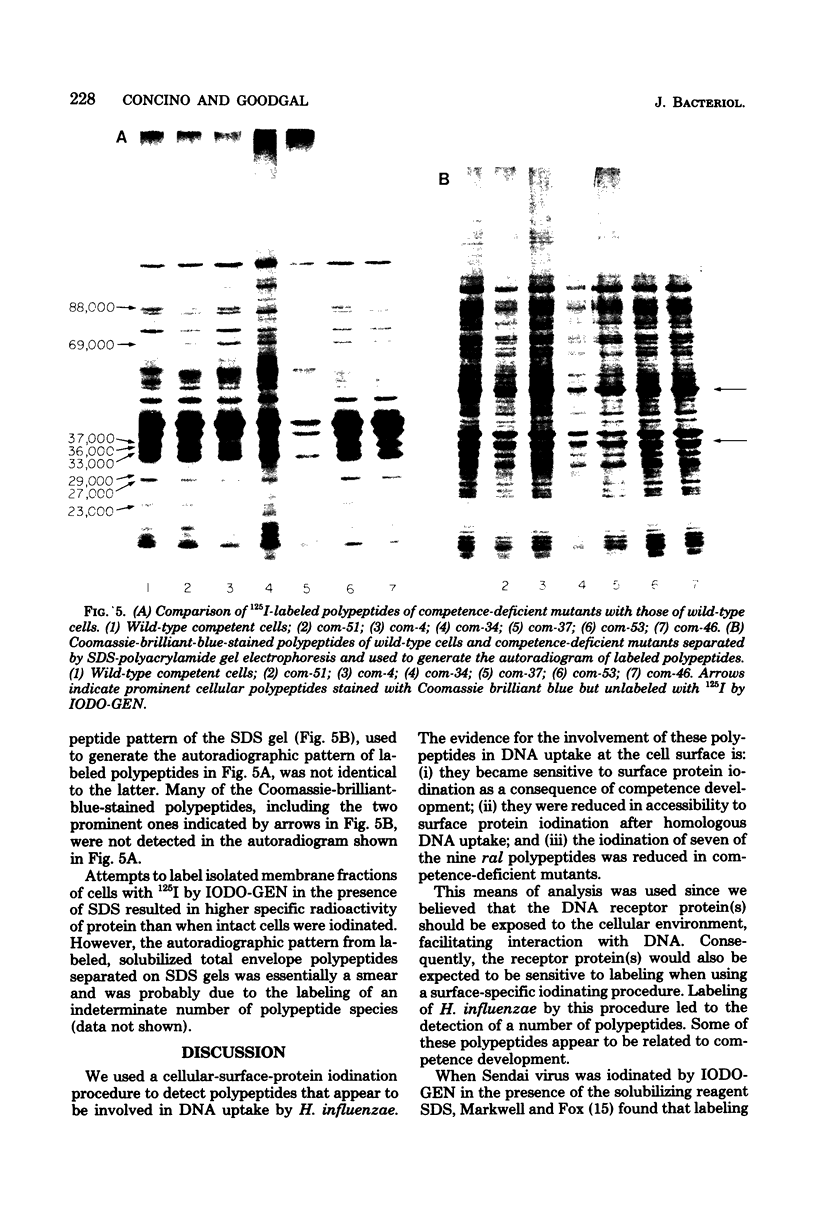
Polypeptides that appear to be involved in competence development and deoxyribonucleic acid (DNA) uptake by Haemophilus influenzae were detected with a surface-specific iodinating reagent 1,3,4,6,-tetrachloro-3 alpha, 6 alpha-diphenylglycoluril. As shown on electrophoretograms, a number of polypeptides became sensitive to 125I protein labeling with the ability of these cells to bind DNA. Of these polypeptides, nine were reduced in their ability to be labeled (ral polypeptides) extensively after the incubation of competent cells with homologous, but not with heterologous, DNA. Iodination of many of these ral polypeptides was reduced in competence-deficient mutants compared with wild-type competent cells. One 125I-labeled polypeptide corresponding to a molecular weight of 29,000 was present at reduced levels in mutants reduced in the ability to bind DNA. Our results suggest that the 29,000-molecular-weight polypeptide corresponds with the ability of H. influenzae to take up DNA and that a complex of proteins is involved in DNA uptake and transformation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNHART B. J., HERRIOTT R. M. PENETRATION OF DEOXYRIBONUCLEIC ACID INTO HEMOPHILUS INFLUENZAE. Biochim Biophys Acta. 1963 Sep 17;76:25–39. [PubMed] [Google Scholar]
- Caster J. H., Goodgal S. H. Competence Mutant of Haemophilus influenzae with Abnormal Ratios of Marker Efficiencies in Transformation. J Bacteriol. 1972 Oct;112(1):492–502. doi: 10.1128/jb.112.1.492-502.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster J. H., Postel E. H., Goodgal S. H. Competence mutants: isolation of transformation deficient strains of Haemophilus influenzae. Nature. 1970 Aug 1;227(5257):515–517. doi: 10.1038/227515a0. [DOI] [PubMed] [Google Scholar]
- DUBE S. K., ROHOLT O. A., PRESSMAN D. IDENTIFICATION OF THE MOST RAPIDLY IODINATING TYROSINE RESIDUE IN ALPHA-CHYMOTRYPSIN. J Biol Chem. 1964 Oct;239:3347–3349. [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Deich R. A., Smith H. O. Mechanism of homospecific DNA uptake in Haemophilus influenzae transformation. Mol Gen Genet. 1980 Feb;177(3):369–374. doi: 10.1007/BF00271475. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Lange R., Willecke K. Competent Bacillus subtilis cultures synthesize a denatured DNA binding activity. Proc Natl Acad Sci U S A. 1975 Jan;72(1):323–327. doi: 10.1073/pnas.72.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Concino M., Gromkova R., Goodgal S. DNA binding activity of vesicles produced by competence deficient mutants of Haemophilus. Biochem Biophys Res Commun. 1979 Apr 13;87(3):764–772. doi: 10.1016/0006-291x(79)92024-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- SPRINGELL P. H. An unreactive tyrosine residue in insulin and the exclusive iodination of the A chain. Nature. 1961 Sep 30;191:1372–1373. doi: 10.1038/1911372a0. [DOI] [PubMed] [Google Scholar]
- STUY J. H., STERN D. THE KINETICS OF DNA UPTAKE BY HAEMOPHILUS INFLUENZAE. J Gen Microbiol. 1964 Jun;35:391–400. doi: 10.1099/00221287-35-3-391. [DOI] [PubMed] [Google Scholar]
- Scocca J. J., Poland R. L., Zoon K. C. Specificity in deoxyribonucleic acid uptake by transformable Haemophilus influenzae. J Bacteriol. 1974 May;118(2):369–373. doi: 10.1128/jb.118.2.369-373.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seon B. K., Roholt O. A., Pressman D. Environments of individual tyrosines in protein. Differences in rates of mono- and diiodination. Biochim Biophys Acta. 1970 Oct 20;221(1):114–118. doi: 10.1016/0005-2795(70)90202-3. [DOI] [PubMed] [Google Scholar]
- Spencer H. T., Herriott R. M. Development of competence of Haemophilus influenzae. J Bacteriol. 1965 Oct;90(4):911–920. doi: 10.1128/jb.90.4.911-920.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrina S. L., Scocca J. J. Haemophilus influenzae periplasmic protein which binds deoxyribonucleic acid: properties and possible participation in genetic transformation. J Bacteriol. 1979 Sep;139(3):1021–1027. doi: 10.1128/jb.139.3.1021-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Habersat M., Scocca J. J. Synthesis of envelope polypeptides by Haemophilus influenzae during development of competence for genetic transformation. J Bacteriol. 1976 Jul;127(1):545–554. doi: 10.1128/jb.127.1.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Scocca J. J. Constitution of the cell envelope of Haemophilus influenzae in relation to competence for genetic transformation. J Bacteriol. 1975 Aug;123(2):666–677. doi: 10.1128/jb.123.2.666-677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]