Abstract
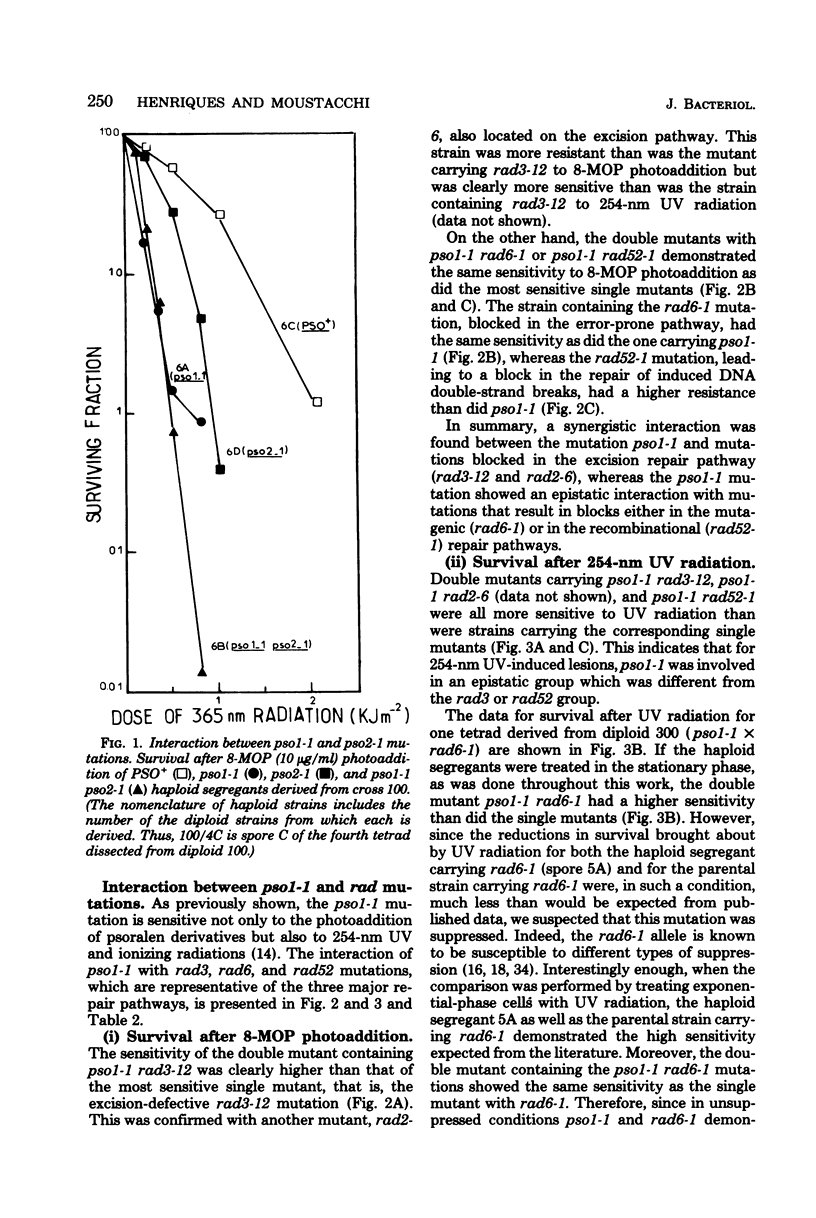
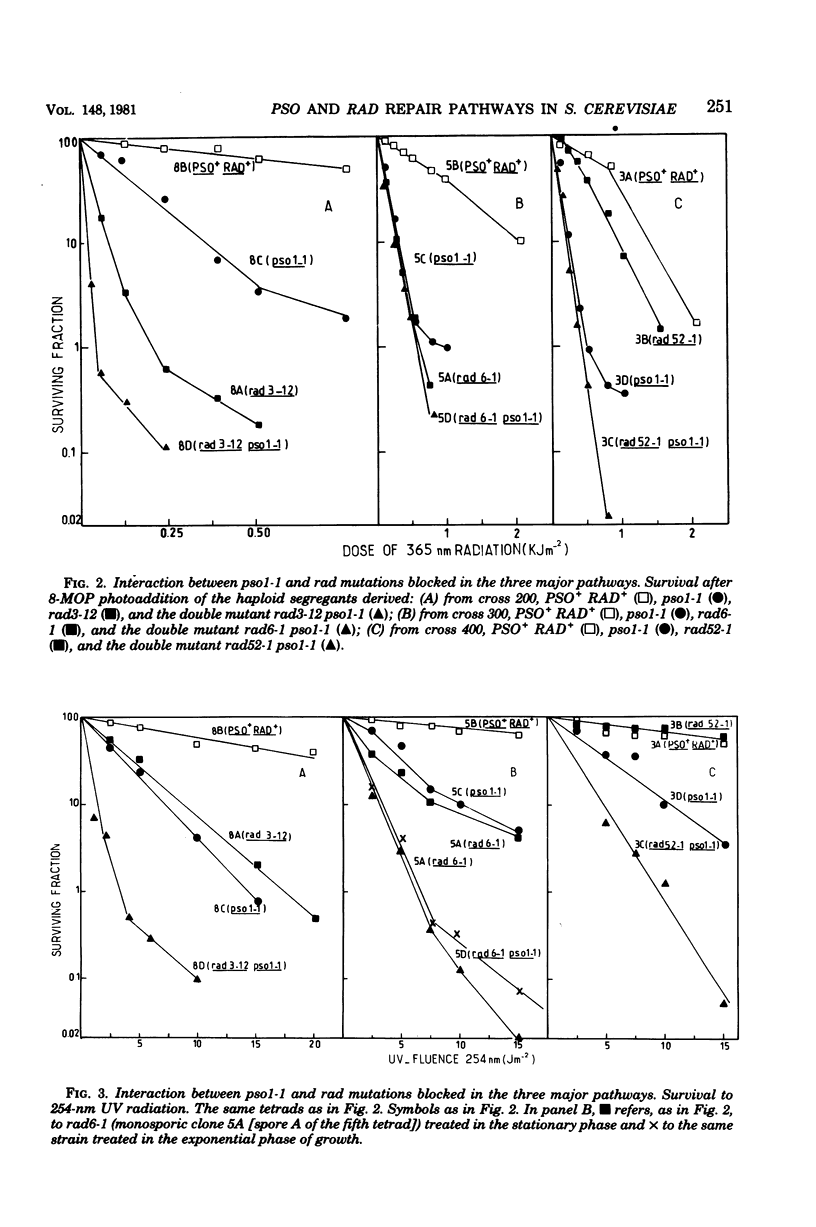
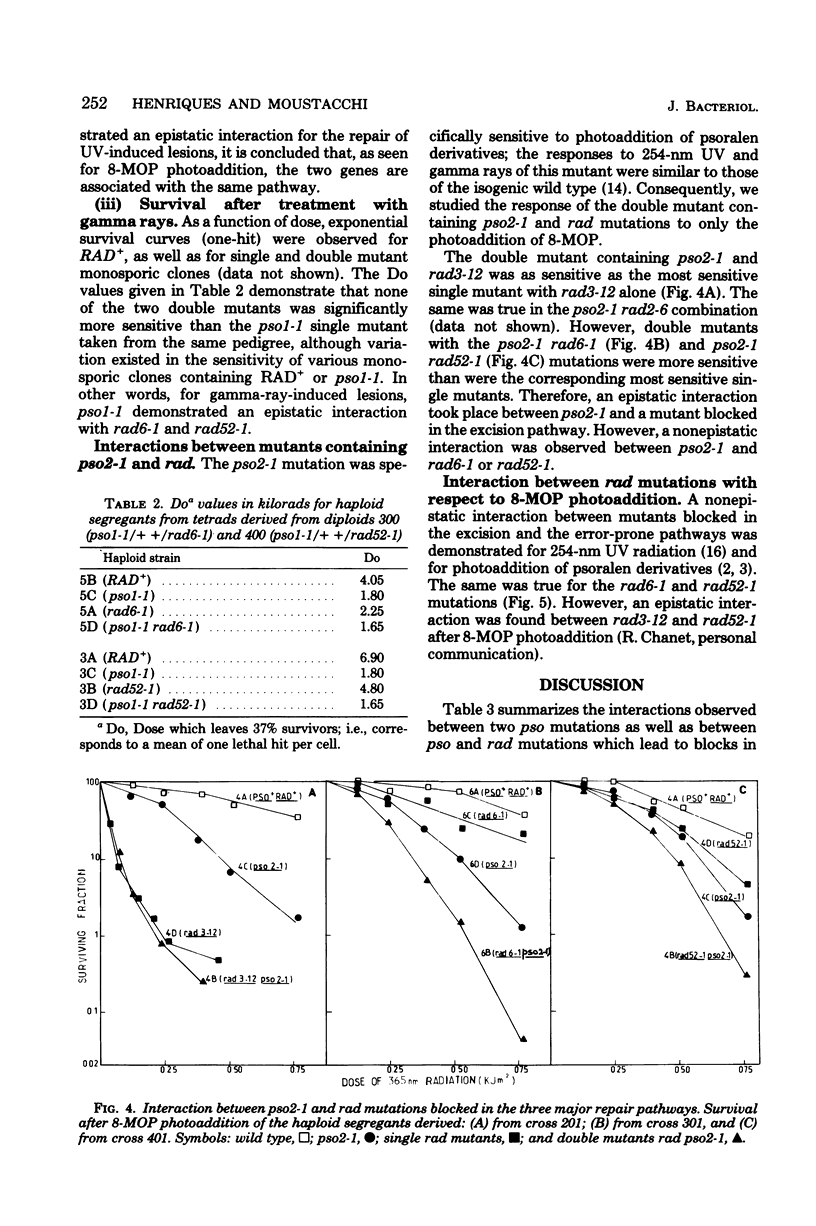
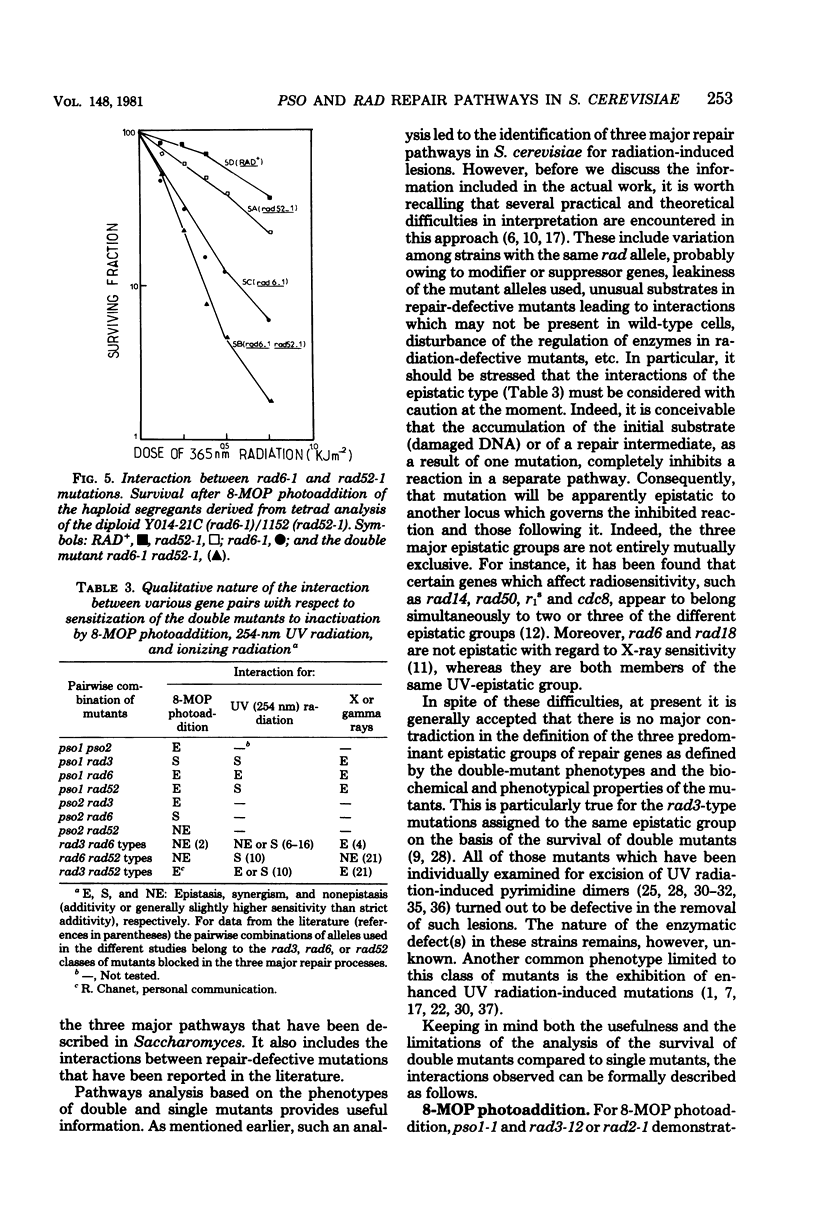
The mode of interaction in haploid Saccharomyces cerevisiae of two pso mutations with each other and with rad mutations affected in their excision-resynthesis (rad3), error-prone (rad6), and deoxyribonucleic acid double-strand break (rad52) repair pathways was determined for various double mutant combinations. Survival data for 8-methoxypsoralen photoaddition, 254-nm ultraviolet light and gamma rays are presented. For 8-methoxypsoralen photoaddition, which induces both deoxyribonucleic acid interstrand cross-links and monoadditions, the pso1 mutation is epistatic to the rad6, rad52, and pso2 mutations, whereas it is synergistic to rad3. The pso2 mutation, which is specifically sensitive to photoaddition of psoralens, is epistatic to rad3 and demonstrates a nonepistatic interaction with rad6 and rad52. rad3 and rad6, as well as rad 6 and rad52, show synergistic interactions with each other, whereas rad 3 is epistatic to rad52. Consequently, it is proposed that PSO1 and RAD3 genes govern steps in the independent pathways. The PSO1 activity leading to an intermediate which is repaired via the three incidence pathways controlled by RAD6, RAD52, and PSO2 genes. Since pso1 interacts synergistically with rad3 and rad52 and epistatically with rad6 after UV radiation, the PSO1 gene appears to belong to the RAD6 group. For gamma ray sensitivity, pso1 is epistatic to rad6 and rad52, which suggests that this gene controls a step which is common to the two other independent pathways.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averbeck D., Laskowski W., Eckardt F., Lehmann-Brauns E. Four radiation sensitive mutants of Saccharomyces. Survival after UV- and x-ray-irradiation as well as UV-induced reversion rates from isoleucine-valine dependence to independence. Mol Gen Genet. 1970;107(2):117–127. doi: 10.1007/BF00333628. [DOI] [PubMed] [Google Scholar]
- Averbeck D., Moustacchi E. 8-Methoxypsoralen plus 365 nm light effects and repair in yeast. Biochim Biophys Acta. 1975 Jul 23;395(4):393–404. doi: 10.1016/0005-2787(75)90063-5. [DOI] [PubMed] [Google Scholar]
- Averbeck D., Moustacchi E., Bisagni E. Biological effects and repair of damage photoinduced by a derivative of psoralen substituted at the 3,4 reaction site: photoreactivity of this compound and lethal effect in yeast. Biochim Biophys Acta. 1978 May 23;518(3):464–481. doi: 10.1016/0005-2787(78)90165-x. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Interactions among genes controlling sensitivity to radiation and alkylation in yeast. Mol Gen Genet. 1973 Sep 12;125(3):197–216. doi: 10.1007/BF00270743. [DOI] [PubMed] [Google Scholar]
- Cassier C., Chanet R., Henriques J. A., Moustacchi E. The effects of three PSO genes on induced mutagenesis : a novel class of mutationally defective yeast. Genetics. 1980 Dec;96(4):841–857. doi: 10.1093/genetics/96.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B., Game J. Repair systems in Saccharomyces. Mutat Res. 1974 Aug;26(4):257–264. doi: 10.1016/s0027-5107(74)80023-0. [DOI] [PubMed] [Google Scholar]
- Eckardt F., Haynes R. H. Induction of pure and sectored mutant clones in excision-proficient and deficient strains of yeast. Mutat Res. 1977 Jun;43(3):327–338. doi: 10.1016/0027-5107(77)90056-2. [DOI] [PubMed] [Google Scholar]
- Game J. C., Cox B. S. Epistatic interactions between four rad loci in yeast. Mutat Res. 1972 Dec;16(4):353–362. doi: 10.1016/0027-5107(72)90203-5. [DOI] [PubMed] [Google Scholar]
- Game J. C., Cox B. S. Synergistic interactions between rad mutations in yeast. Mutat Res. 1973 Oct;20(1):35–44. doi: 10.1016/0027-5107(73)90095-x. [DOI] [PubMed] [Google Scholar]
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Henriques J. A., Moustacchi E. Isolation and characterization of pso mutants sensitive to photo-addition of psoralen derivatives in Saccharomyces cerevisiae. Genetics. 1980 Jun;95(2):273–288. doi: 10.1093/genetics/95.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. A., Brendel M., Haynes R. H. Supersensitive double mutants in yeast. Mol Gen Genet. 1970;107(4):376–378. doi: 10.1007/BF00441200. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. UV mutagenesis in radiation-sensitive strains of yeast. Genetics. 1976 Feb;82(2):207–232. doi: 10.1093/genetics/82.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Stewart J. W., Sherman F., Christensen R. Specificity and frequency of ultraviolet-induced reversion of an iso-1-cytochrome c ochre mutant in radiation-sensitive strains of yeast. J Mol Biol. 1974 May 5;85(1):137–162. doi: 10.1016/0022-2836(74)90134-x. [DOI] [PubMed] [Google Scholar]
- Lemontt J. F. Pathways of ultraviolet mutability in Saccharomyces cerevisiae. I. Some properties of double mutants involving uvs9 and rev. Mutat Res. 1971 Dec;13(4):311–317. doi: 10.1016/0027-5107(71)90041-8. [DOI] [PubMed] [Google Scholar]
- Magaña-Schwencke N., Averbeck D., Pegas-Henriques J. A., Moustacchi E. Absence de pontages inter-chaînes dan l'ADN traité par le 3-carbéthoxypsoralène et une irradiation à 365 nm. C R Seances Acad Sci D. 1980 Sep 15;291(2):207–210. [PubMed] [Google Scholar]
- McKee R. H., Lawrence C. W. Genetic analysis of gamma-ray mutagenesis in yeast. III. Double-mutant strains. Mutat Res. 1980 Mar;70(1):37–48. doi: 10.1016/0027-5107(80)90056-1. [DOI] [PubMed] [Google Scholar]
- Moustacchi E. Cytoplasmic and nuclear genetic events induced by UV light in strains of Saccharomyces cerevisiae with different UV sensitivities. Mutat Res. 1969 Mar-Apr;7(2):171–185. doi: 10.1016/0027-5107(69)90029-3. [DOI] [PubMed] [Google Scholar]
- Nakai S., Matsumoto S. Two types of radiation-sensitive mutant in yeast. Mutat Res. 1967 Mar-Apr;4(2):129–136. doi: 10.1016/0027-5107(67)90064-4. [DOI] [PubMed] [Google Scholar]
- Prakash L., Prakash S. Three additional genes involved in pyrimidine dimer removal in Saccharomyces cerevisiae: RAD7, RAD14 and MMS19. Mol Gen Genet. 1979 Nov;176(3):351–359. doi: 10.1007/BF00333097. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in nuclear and mitochondrial DNA of yeast irradiated with low doses of ultraviolet light. J Mol Biol. 1975 Nov 15;98(4):781–795. doi: 10.1016/s0022-2836(75)80010-6. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in radiation-sensitive mutants rad3, rad4, rad6 and rad9 of Saccharomyces cerevisiae. Mutat Res. 1977 Oct;45(1):13–20. doi: 10.1016/0027-5107(77)90038-0. [DOI] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A. Genetic control of radiation sensitivity in Saccharomyces cerevisiae. Genetics. 1969 Jul;62(3):519–531. doi: 10.1093/genetics/62.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Setlow J. K. Repair of pyrimidine dimer damage induced in yeast by ultraviolet light. J Bacteriol. 1972 Mar;109(3):979–986. doi: 10.1128/jb.109.3.979-986.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. J. Removal of pyrimidine dimers from Saccharomyces cerevisiae nuclear DNA under nongrowth conditions as detected by a sensitive, enzymatic assay. Mutat Res. 1978 Apr;50(1):43–56. doi: 10.1016/0027-5107(78)90059-3. [DOI] [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Cox B. S. Ultraviolet mutagenesis studies of [psi], a cytoplasmic determinant of Saccharomyces cerevisiae. Genetics. 1980 Jul;95(3):611–630. doi: 10.1093/genetics/95.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unrau P., Wheatcroft R., Cox B. S. The excision of pyrimidine dimers from DNA of ultraviolet irradiated yeast. Mol Gen Genet. 1971;113(4):359–362. doi: 10.1007/BF00272336. [DOI] [PubMed] [Google Scholar]
- Waters R., Moustacchi E. The disappearance of ultraviolet-induced pyrimidine dimers from the nuclear DNA of exponential and stationary phase cells of Saccharomyces cerevisiae following various post-irradiation treatments. Biochim Biophys Acta. 1974 Jul 24;353(4):407–419. doi: 10.1016/0005-2787(74)90048-3. [DOI] [PubMed] [Google Scholar]
- Zakharov I. A., Kozina T. N., Fedorova I. V. Effets de mutations vers la sensibilité au rayonnement ultraviolet chez la levure. Mutat Res. 1970 Jan;9(1):31–39. doi: 10.1016/0027-5107(70)90068-0. [DOI] [PubMed] [Google Scholar]



