Abstract
Synthetic coat protein complex I (COPI)-coated vesicles form spontaneously from large (≈300 nm in diameter), chemically defined liposomes incubated with coatomer, Arf1p, and guanosine 5′-[γ-thio]triphosphate. Coated vesicles are 40–70 nm in diameter, approximately the size of COPI vesicles formed from native membranes. The formation of COPI-coated buds and vesicles and the binding of Arf1p to donor liposomes depends on guanosine 5′-[γ-thio]triphosphate. In contrast to the behavior of the COPII coat, coatomer binds to liposomes containing a variety of charged or neutral phospholipids. However, the formation of COPI buds and vesicles is stimulated by acidic phospholipids. In the absence of Arf1p, coatomer binds to liposomes containing dioleoylphosphatidic acid as a sole acidic phospholipid to form large coated surfaces without forming COPI-coated buds or vesicles. We conclude that Arf1p-GTP and coatomer comprise the minimum apparatus necessary to create a COPI-coated vesicle.
Protein transport between different compartments in a cell is achieved by coated vesicles. Three different coats have been identified to date: Clathrin coated vesicles are responsible for transport between the trans-Golgi and the plasma membrane and in the endocytic pathway (1); coat protein complex II (COPII) mediates anterograde transport from the endoplasmic reticulum (ER) to the Golgi (2, 3); and COPI-coated vesicles are involved in anterograde and retrograde transport between the ER and the Golgi and within the Golgi (refs. 4 and 5; A.S. and R.S., unpublished work). The assembly unit of the COPI coat comprises the heptameric protein complex called coatomer (α-COP, β-COP, β′-COP, γ-COP, δ-COP, ɛ-COP, ζ-COP), and coat assembly is governed by a small GTP-ase Arf1p (for review, see refs. 3 and 6–8).
The formation of the COPI vesicle begins when Arf1p is recruited to a target membrane through an interaction with a nucleotide exchange catalyst that converts Arf1p-GDP to the active species Arf1p-GTP (9, 10). Activated Arf1p allows the recruitment of coatomer to a membrane in which coated buds and vesicles form and capture proteins for transport. The exact role of Arf1p in this process is controversial. The conventional view is that activated Arf1p serves as a direct membrane anchor for coatomer (11). This assertion is supported in part by the observation that Arf1p–guanosine 5′-[γ-thio]triphosphate (GTP-γ-S) becomes concentrated in COPI-coated vesicles (12, 13). However, because Arf1p-GTP (or GTP-γ-S) is also an activator of phospholipase D (PLD), an alternative view is that the activation of PLD results in the hydrolysis of phosphatidylcholine to phosphatidic acid (PA), creating a favorable binding environment for coatomer (14). According to this view, Arf1p-GTP may act indirectly to promote COPI-coated vesicle formation.
Coatomer recruitment to membranes also may be driven by the recognition of protein cargo molecules. The C-terminal KKXX-motif of membrane proteins is known to interact with coatomer and to engage COPI vesicles in retrograde transport from the Golgi to the ER. However, although subunits of the anterograde carrier, COPII, also interact directly with protein cargo leaving the ER membrane (15), the COPII coat is capable of forming synthetic COPII vesicles by budding from pure phospholipid vesicles (16).
We developed a direct test of the role of PLD and protein cargo in the formation of COPI buds and vesicles. Our evidence supports the view that Arf1p serves directly in the budding process whereas membrane protein cargo is not essential.
MATERIALS AND METHODS
Materials.
Most of the phospholipids and their derivatives were purchased from Avanti Polar Lipids. Phosphatidylinositol 4-phosphate (PIP), phosphatidylinositol 4,5-bisphosphate (PIP2), cytidine 5′-diphosphate-diacylglycerol, ergosterol, and other reagents, unless specified, were obtained from Sigma. Proteins resolved by SDS/PAGE were stained with SYPRO Red (Molecular Probes). Escherichia coli BL21(DE3) strains, which coexpress yeast N-myristoyltransferase and either wild-type or dominant activated yeast Arf1p, were provided by R. Kahn (Emory Univ.). N-myr-yArf1p (wild-type) and N-myr-yArf1p (Q71L) were purified as described (17). Yeast coatomer was prepared as described by Hosobuchi et al. (18).
Preparation of Liposomes and Binding of COPI Proteins.
Liposomes were made from a mixture containing 80% (by weight) phospholipids and 20% ergosterol. Dried lipids were hydrated at 2.3 mg/ml and were extruded five times through a 400-nm polycarbonate filter (Poretics) to prepare liposomes as described (16). The phospholipid mix contained, unless indicated, 49 mol % dioleoylphosphatidylcholine (DOPC), 21 mol % dioleoylphosphatidylethanolamine (DOPE), 8 mol % phosphatidylinositol (PI) from soybean, 8 mol % dioleoylphosphatidylserine (DOPS), 5 mol % dioleoylphosphatidic acid (DOPA), 2.2 mol % PIP, 0.8 mol % PIP2, 2 mol % cytidine 5′-diphosphate–diacylglycerol, 2 mol % 7-(nitrobenz 2-oxa-1,3 diazol-4yl)-(NBD-) derivative of DOPE, and 2 mol % 1-oleoyl-2-NBD-N-aminododecanoyl-sn-glycero-3-phosphocholine (COPII-mix; ref. 16). A portion (11 μl) of this mixture was incubated with 20 μg Arf1p or Arf1Q71Lp in 25 mM Hepes (pH 7.4), 0.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 100 mM NaCl, and 0.14 mM GTP-γ-S or guanosine 5′-[β-thio] diphosphate for 1 h at 30°C. The reactions were chilled on ice, and 37.5 μg of coatomer was added. After 15 min, the samples were fixed and processed for electron microscopy as described below.
For sedimentation analysis, liposomes incubated with a combination of Arf1Q71Lp or Arf1p, coatomer, and nucleotide were loaded on top of a linear sucrose gradient (0.2–1.8 M sucrose) in 20 mM Hepes (pH 7.4), 150 mM potassium acetate, 5 mM MgCl2, and 250 mM sorbitol. The gradient was centrifuged for 16 h at 55,000 rpm in a Beckman TLS55 rotor. Fractions (23 × 100 μl) were collected from the top. For analytical purposes, the fractions were collected into a microtiter plate, and the fluorescence of the NBD-phospholipids in each fraction was analyzed by using a storm 860 image analyzer (Molecular Dynamics). In preparative scale reactions, fractions from four identical gradients were combined and processed for electron microscopy as described earlier (16).
For flotation analysis, liposomes were made with various combinations of phospholipids. The resulting liposomes were incubated with Arf1p and coatomer in the presence of guanine nucleotide as described above. Liposomes were recovered by flotation centrifugation, and liposome-bound proteins were analyzed by SDS/PAGE and SYPRO Red staining. COPII binding to these liposomes was carried out as described (16).
Electron Microscopy.
After the binding reaction, either total reactions or sucrose gradient-separated liposomes were fixed with 2% glutaraldehyde and 1% osmium tetroxide in cacodylate buffer for 1 h on ice. Fixed samples were sedimented by centrifugation at 100,000 × g for 45 min. Pellets were contrasted with tannic acid and were embedded in Epon. Thin sections were contrasted further with uranyl acetate and lead citrate and were photographed in a Philips CM10 electron microscope (Philips Electron Instruments, Eindhoven, Netherlands). Photographs taken at a calibrated magnification were printed on paper. The size and number of the different species of liposomes were assessed on the enlarged photographic prints. The size was measured with a metallic ruler calibrated in 1/2 mm.
RESULTS
COPI-Coated Vesicles Can Be Formed from Synthetic Liposomes.
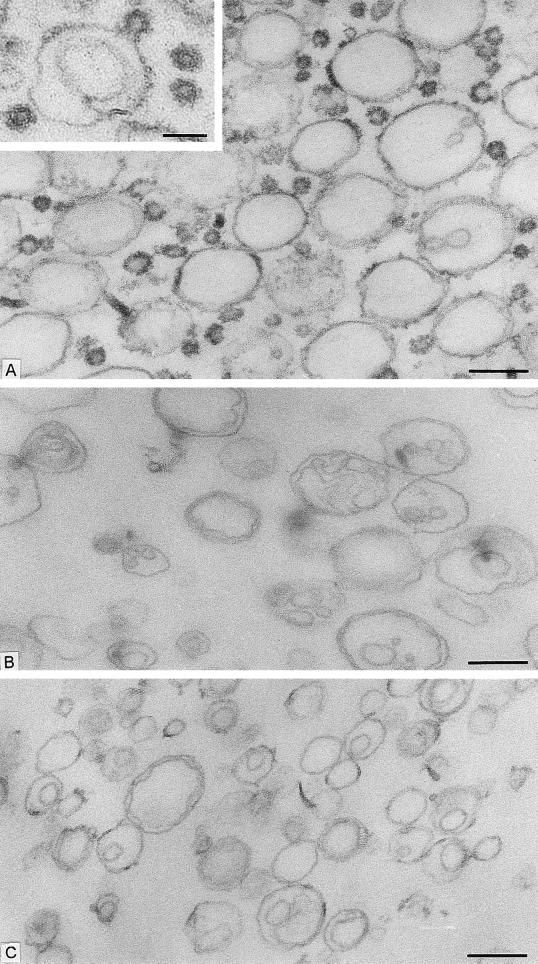
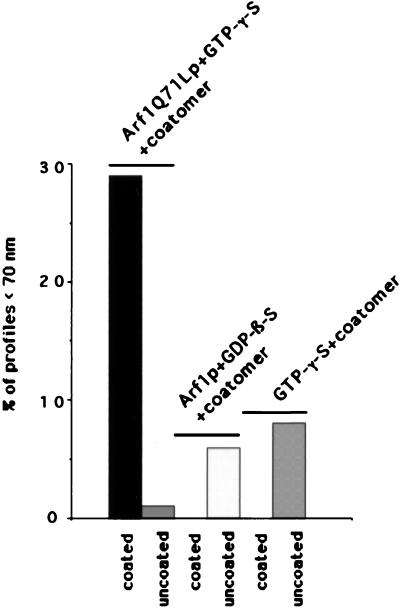
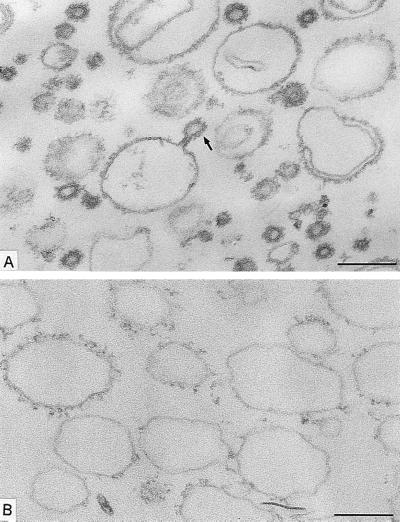
We started with the hypothesis that COPI, like COPII, would be capable of budding vesicles from a liposome of defined phospholipid composition. Liposomes were made from a mixture of phospholipids that allow the recruitment of Sar1p and COPII proteins in a nucleotide-dependent manner (16). This phospholipid composition is similar to that of microsomal membranes from yeast (19). Liposomes were incubated with myristylated, dominant active mutant Arf1p (Arf1Q71Lp), and GTP-γ-S under conditions that allow spontaneous nucleotide exchange and thus insertion of Arf1p-GTP-γ-S into the membranes (20). On the addition of coatomer at low temperature (or at physiological temperature), COPI coats were observed on patches, buds, and vesicles (Fig. 1A). An example of a coated bud is shown in the Fig. 1A Inset. In incubations with coatomer and Arf1Q71Lp, ≈30% of the closed membrane profiles had a diameter <70 nm, and almost all of them were fully coated (Fig. 2). Increasing levels of Arf1Q71Lp and GTP-γ-S led to more abundant production of <70-nm coated vesicles (data not shown). In contrast, only uncoated liposomes were observed in incubations with GTP-γ-S but lacking Arf1p (Fig. 1B) or in the presence of coatomer, Arf1p, and GDP-β-S (Fig. 1C). In these cases, only 6% and 8%, respectively, of the closed membrane profiles had a diameter <70 nm (Fig. 2). These results suggest that Arf1p-GTP-γ-S plays an essential role in the formation of coatomer-coated vesicles whereas integral membrane cargo proteins do not.
Figure 1.
Formation of COPI-coated vesicles from liposomes consisting of 49 mol % DOPC, 21 mol % DOPE, 8 mol % PI, 8 mol % DOPS, 5 mol % DOPA, 2.2 mol % PIP, 0.8 mol % PIP2, 2 mol % cytidine 5′-diphosphate–diacylglycerol, 2 mol % NBD- derivative of DOPE, and 2 mol % 1-oleoyl-2-NBD-N-aminododecanoyl-sn-glycero-3-phosphocholine. Liposomes were incubated with combinations of Arf1p, coatomer, and nucleotides. After incubation, membrane morphology was analyzed by conventional electron microscopy. (A) Liposomes were incubated with Arf1Q71L p and GTP-γ-S for 1 h at 30°C, followed by the addition of coatomer and further incubation for 15 min at 4°C. A mixture of large, partially coated and small, vesicle-like coated liposome profiles are present. (Inset) A coated bud-like element on a large liposome. Free coated vesicular profiles also are seen. (B) Liposomes were incubated with GTP-γ-S for 1 h at 30°C and then with coatomer for 15 min at 4°C without Arf1p. No coats are present on the liposome profiles. (C) Liposomes were incubated with Arf1p and GDP-β-S for 1 h at 30°C. After addition of coatomer, the mixture was kept at 4°C for 15 min. The liposome population is uncoated. [Bars = 200 nm (A–C) and = 100 nm (Inset).]
Figure 2.
Size distribution of liposomes after incubation with COPI-coat components and nucleotides. Liposomes were incubated either in the presence of Arf1Q71Lp and GTP-γ-S, Arf1p and GDP-β-S, or GTP-γ-S alone for 1 h at 30°C. Coatomer was added to all of the samples and was incubated for 15 min at 4°C. The percentage of coated or uncoated, <70-nm, vesicle-like profiles was evaluated on electron microscopy pictures of 120 liposomes in each condition. The mean diameter of the liposomes after incubation was 136 ± 5nm, 152 ± 5 nm, and 159 ± 6 nm (SEM), respectively.
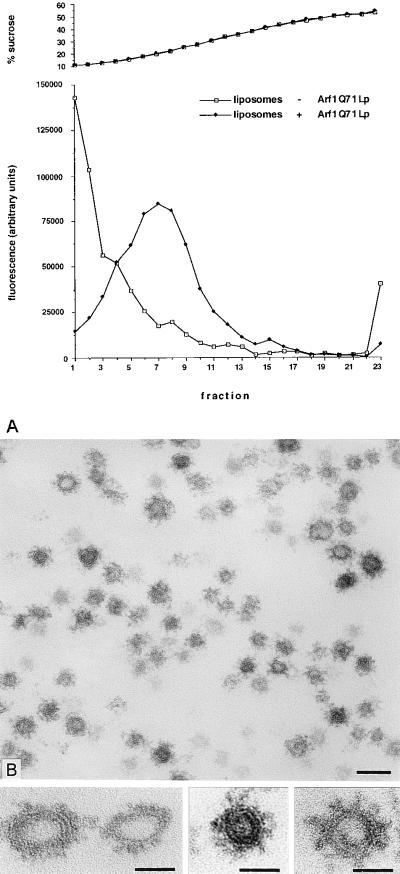
Further characterization of the COPI-coated synthetic vesicles was achieved with an enriched fraction isolated by sedimentation to equilibrium on a linear sucrose gradient. The fractionation of the lipids on this gradient was monitored by measuring the fluorescence of 1-oleoyl-2-NBD-N-aminododecanoyl-sn-glycero-3-phosphoethanolamine (NBD-PE) and 1-oleoyl-2-NBD-N-aminododecanoyl-sn-glycero-3-phosphocholine included in the starting liposome material. Most of the fluorescence was recovered in fractions 4–11 (Fig. 3A), with a leading shoulder sedimenting in fractions 14–16. The sedimentation of lipids to fractions 4–11 and 14–16 depended on the addition of Arf1Q71Lp (Fig. 3A, compare closed and open symbols). The liposomes in fractions 4–11 represent a mixture of larger, partially coated liposomes. Fractions 14–16 contained mostly small vesicles (50 ± 16 nm; n = 240) fully coated with a spike-like coat attributable to Arf1Q71Lp and coatomer (Fig. 3 B and Bottom). Similar results were obtained when Arf1Q71Lp was replaced by Arf1p (data not shown).
Figure 3.
Enrichment of COPI-coated vesicles. (A) Distribution of lipids on a sucrose-density gradient after a binding reaction followed by centrifugation to equilibrium. (B) COPI-coated vesicles present in fractions 14–16 of the sucrose-density gradient. The mean size of the coated vesicles was 50 ± 16 nm (SD). (Bar = 100 nm.) (Bottom) Gallery of coated vesicles in equatorial section. (Bars = 50 nm.)
Lipid Requirements for the Formation of COPI-Coated Vesicles.
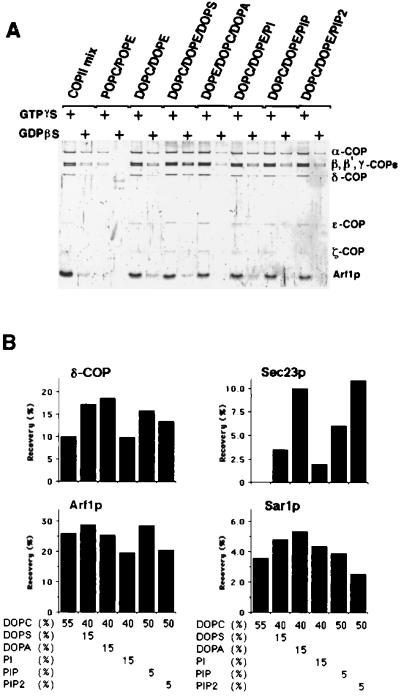
We next explored the specific phospholipid requirements for recruitment of coatomer and Arf1p and for the budding of COPI vesicles. Liposomes from simple lipid mixtures were incubated in the presence of GTP-γ-S or GDP-β-S with Arf1p and coatomer. Coatomer and Arf1p bound to liposomes were recovered by flotation on a sucrose density gradient. Lipid recovery was monitored by NBD-PE fluorescence, and coatomer and Arf1p binding to liposomes were evaluated by SDS/PAGE. Liposomes made from DOPC and DOPE, which contain unsaturated fatty acyl side chains, recruited Arf1p and coatomer essentially as well as the more complex mixture of phospholipids found to be optimal for recruitment of COPII (ref. 16; Fig. 4A and B; COPII mix; DOPC/DOPE). We found that no PA was formed during the incubation period (data not shown). Binding of both coatomer and Arf1p was reduced greatly to liposomes composed of phospholipids carrying one saturated and one unsaturated fatty acid [Fig. 4A, 1-palmitoyl-2-oleoyl-phosphatidyl choline (POPC)/1-palmitoyl-2-oleoyl-phosphatidyl ethanolamine (POPE)]. This result suggests that the lower fluidity of the POPC/POPE liposomes may prevent the insertion or stable retention of Arf1p-GTP in the lipid bilayer. Inclusion of 15 mol % of the acidic phospholipid DOPS in the DOPC/DOPE mixture increased the binding of coatomer without changing the binding efficiency of Arf1p. However, coatomer binding in the presence of GDP-β-S also was increased. Substitution of DOPE by 15 mol % DOPA or 5 mol % PIP in DOPC/DOPE liposomes yielded a similar increase in coatomer binding. In contrast, supplementation of liposomes with PI or PIP2 did not enhance the recruitment of Arf1p and coatomer. These lipid specificities were distinct from those displayed by subunits of COPII (ref. 16 and Fig. 4B). Although Sar1p was recruited to all of the liposomes tested, Sec23/24p (Fig. 4B) and Sec13/31p (data not shown) only were bound efficiently to liposomes supplemented with DOPA or PIP2.
Figure 4.
Lipid requirement for binding of COPI and COPII components. (A) Liposomes composed of various combinations of phospholipids were tested for binding of COPI proteins in the presence of 0.1 mM GTP-γ-S or 0.1 mM GDP-β-S. Liposomes were floated through a sucrose cushion as described in Materials and Methods. Liposome-bound proteins were separated by SDS/PAGE, were stained with SYPRO-Red, and visualized with a storm860 image analyzer. COPII mix, liposomes used for COPII budding (ref. 16; K.M., unpublished material); POPC/POPE, liposomes made of 55 mol % 1-palmitoyl-2-oleoyl-phosphatidylcholine, 44 mol % 1-palmitoyl-2-oleoyl-PE, and 1 mol % of NBD-PE; DOPE/DOPC, liposomes made of 55 mol % DOPC, 44 mol % DOPE, and 1 mol % NBD-PE; DOPC/DOPE/DOPS, liposomes made of 40 mol % DOPC, 44 mol % DOPE, 15 mol % DOPS, and 1 mol % NBD-PE; DOPC/DOPE/DOPA, liposomes made of 40 mol % DOPC, 44 mol % DOPE, 15 mol % DOPA, and 1 mol % NBD-PE; DOPC/DOPE/PI, liposomes made of 40 mol % DOPC, 45 mol % DOPE, and 15 mol % PI from soybean; DOPC/DOPE/PIP, liposomes made of 50 mol % DOPC, 45 mol % DOPE, and 5 mol % PIP from bovine brain; DOPC/DOPE/DOPS, liposomes made of 50 mol % DOPC, 45 mol % DOPE, and 5 mol % PIP2 from bovine brain. (B) Comparison of the binding of coatomer, Arf1p, and COPII proteins to liposomes made of various mixtures of phospholipids. Protein-liposome complexes were recovered from fractions floated in a sucrose density gradient. The yield of Arf1p and δ-COP from incubations conducted in the presence of GTP-γ-S and of Sar1p and Sec23p from incubations containing guanosine 5′-[β,γ- imidylyl]triphosphate are shown.
The influence of lipid composition on coat and bud formation was measured ultrastructurally. The DOPC/DOPE mixtures gave rise to partially coated larger vesicles, and only a modest level of coated vesicles smaller than 70 nm were observed (Table 1). Budding to form COPI vesicles was stimulated by supplementation of liposomes with phosphatidylserine (PS) or with PS and PA and depended on the presence of GTP-γ-S (Table 1). However, liposomes containing PS also fragmented to produce uncoated vesicles in incubations that contained GDP-β-S. This potential instability was reduced in the liposomes supplemented with PA. These results suggest that Arf1p-GTP-γ-S is essential for COPI vesicle formation even on liposomes that contain an acidic phospholipid such as PA.
Table 1.
Size distribution of liposomes after incubation with COPI proteins and nucleotides
| Phosphoplipid composition of the liposomes | GTP[γS] | GDP[βS] | Diameter, nm, mean ± SEM | Liposomes or vesicles <70 nm, % of profiles
|
n | |
|---|---|---|---|---|---|---|
| Coated | Noncoated | |||||
| 55 mol % DOPC, 44 mol % DOPE, | + | 169 ± 9 | 18 | 2 | 120 | |
| 1 mol % NBD-PE | + | 260 ± 14 | 0 | 5 | 120 | |
| 40 mol % DOPC, 44 mol % DOPE, | + | 136 ± 6 | 28 | 0 | 120 | |
| 15 mol% DOPS, 1 mol % NBD-PE | + | 141 ± 4 | 1 | 13 | 120 | |
| 40 mol % DOPC, 41.5 mol % DOPE, | + | 120 ± 5 | 32 | 1 | 120 | |
| 15 mol % DOPS, 2.5 mol % DOPA, 1 mol % NBD-PE | + | 202 ± 9 | 1 | 8 | 120 | |
| 40 mol % DOPC, 44 mol % DOPE, | + | 129 ± 7 | 40 | 1 | 120 | |
| 15 mol % DOPA, 1 mol % NBD-PE | + | 213 ± 11 | 5 | 7 | 120 | |
The liposomes were incubated with Arf1Q71Lp and guanine nucleotides for 1 h at 30°C. Coatomer was added to all samples, and vesicle formation was allowed to occur for 15 min at 30°C and 15 min at 4°C. The reaction mixtures were fixed, pelleted, and processed for electron microscopy. Randomly chosen areas from ultrathin sections were used for the analysis.
Arf1p Is Indispensable for the Formation of COPI-Coated Vesicles.
Ktistakis et al. (14) reported that COPI-coated vesicles could be formed from liposomes containing PA even in the absence of Arf1p. In contrast, we did not observe small coated vesicles (<70 nm) when PA-containing liposomes were incubated with Arf1Q71Lp and GDP-β-S. To rule out the possibility that Arf1p-GDP inhibited COPI budding, we repeated the experiments with the DOPE/DOPC/DOPA liposomes by using GTP-γ-S and coatomer but without Arf1p. As before, incubations with Arf1Q71Lp, GTP-γ-S, and coatomer produced small COPI vesicles (Fig. 5A). However, omission of Arf1p resulted in coated liposomes but few, if any, coated vesicles (Fig. 5B). The coat on liposomes appeared less dense than on COPI vesicles, suggesting that Arf1p may cluster coatomer to reach a “critical concentration” necessary for bud formation. The appearance of a coat on liposomes in incubations that lack Arf1p-GTP-γ-S may be enhanced by an unphysiologically high level of acidic phospholipids. This structure was not apparent on liposomes containing a more physiologic mix of phospholipids (e.g., Fig. 1 B and C).
Figure 5.
Arf1p is essential for the formation of COPI-coated vesicles. Liposomes consisting of 40 mol % DOPC, 44 mol % DOPE, 15 mol % DOPA, and 1 mol % NBD-PE were incubated for 1 h at 30°C with Arf1Q71Lp and GTP-γ-S (A) or GTP-γ-S (B). Coatomer was added to both samples, and binding was allowed to occur for 15 min at 30°C and 15 min at 4°C. Profiles of 120 liposomes of each condition were analyzed. In A, coated vesicle-like profiles are mixed with large liposomes showing coated segments, one in the form of a coated bud-like profile (arrow). In this incubation, 27% of the liposomes were <70 nm and were coated; only 1% of <70-nm profiles are uncoated. (B) Mostly large liposomes with loose coats were present. In this incubation, 50% of the >70-nm profiles had coated segments; only 5% of the liposomes were <70 nm, and 80% of those were uncoated. The mean diameter of the liposomes was 145 ± 7 nm in A and 296 ± 14 nm (SEM) in B. The bars in (A and B) represent 200 nm.
DISCUSSION
Coatomer and Arf1p comprise the minimum cytosolic set of proteins necessary for COPI vesicle budding from the Golgi apparatus (21, 22). We now have extended this conclusion to demonstrate that no membrane proteins are essential for this process. Liposomes of simple and defined compositions are sufficient to create a platform for the binding of coatomer and Arf1p and for the production of synthetic COPI vesicles that approximate in size the authentic vesicles formed from Golgi membranes. Thus, as was shown for COPII (16), and also was shown recently for the clathrin coat (23), COPI subunits assemble in a regular manner that dictates the size and shape of a coated vesicle. In each case, acidic phospholipids promote coat protein recruitment. However, each coat differs in the specificity of the acidic lipid requirement and in the dependence for vesicle formation on the presence of a small GTP-binding protein. The major subunits of COPII bind to liposomes and bud vesicles only in the presence of Sar1p-guanosine 5′[β,γ-imidylyl]triphosphate (16). Clathrin vesicles form from acidic liposomes even in the absence of GTP-binding protein (23). Here, we have shown that coatomer binds to acidic liposomes, but, without Arf1p-GTP-γ-S, COPI vesicle budding is not observed. The degree to which each of these coats sequesters acidic phospholipids has not been addressed.
Integral membrane proteins may augment budding or may replace the requirement for acidic phospholipids in coated vesicle budding from native membranes. Retrieval motifs such as the C-terminal KKXX-signal on certain recycling ER membrane proteins, and signals on SNARE molecules or retrograde receptors (e.g., Erd2p), interact with coat proteins passively to become enriched in vesicles or actively to facilitate coat assembly (24–26). Nucleotide exchange catalysts such as Sec12p for Sar1p (27) or Gea1p/Gea2p for Arf1p (28, A.S., J. M. Hermann, A. Peyroche, C. L. Jackson, and R.S., unpublished data) direct the localization of coat assembly to the proper target membrane. However, our results suggest that these proteins serve no intrinsic role in vesicle morphogenesis.
Synthetic COPI buds and vesicles form nearly equally well on liposomes composed of DOPC/DOPE/DOPS or DOPC/DOPE/DOPA. However, PS-liposomes appear to fragment into uncoated vesicles in incubation conditions that diminish Arf1p binding (GDP-β-S). PA-liposomes appear to remain more stable, allowing a somewhat more proficient formation of COPI vesicles. COPII proteins do not bind to DOPC/DOPE liposomes, and combinations that include PS have only a limited capacity for this coat (ref. 16 and Fig. 4B). Membrane regional differences in lipid head group composition, particularly an enrichment of PS in later compartments of the secretory pathway (19), may facilitate the localization of COPII to the ER and of COPI to the Golgi and endosomes.
Acyl chain saturation also influences coat recruitment and vesicle budding. DOPC/DOPE vesicles, which contain unsaturated fatty acyl side chains, allow coat recruitment whereas POPC/POPE vesicles, which contain one saturated and one unsaturated fatty acid, do not. Thus, the reduced acyl chain mobility of the POPC/POPE lipid phase, particularly at the low temperature of our coatomer binding and budding reactions (4°C), may limit the ability of N-myr-Arf1p to be retained in a membrane-embedded form suitable for coat recruitment. Similar acyl chain saturation influences were noted in COPII subunit binding to liposomes (16).
Mammalian Arf1p-GTP stimulates PLD, which acts primarily on phosphatidylcholine to form PA (29). In this manner, Arf1p may act indirectly to create an acidic phospholipid conducive to coatomer binding and hence for COPI vesicle formation (14). In contrast, our results show clearly that liposomes containing PA bind coatomer and form a visible coat but do not form COPI vesicles by a budding process unless Arf1p-GTP-γ-S also is bound to the membrane. However, our experiments were performed with yeast proteins, and yeast Arf1p has not been reported to stimulate PLD. Furthermore, the classic PLD in yeast is encoded by SPO14, which is not essential for mitotic growth and thus is not required for secretion (30). The mammalian and yeast systems could differ in the degree to which Arf1p is coupled to COPI vesicle formation. Given the evolutionary conservation of traffic proteins and coat subunits, this explanation seems unlikely. Instead, we suggest that the experiments used to show coatomer binding to liposomes in the absence of Arf1p used small liposomes that simply bound COPI proteins without creating coated vesicles by a budding mechanism (14). Perhaps Arf1p serves both to enhance the pool of PA that facilitates coatomer binding to membranes and to direct the polymerization of the coat into a budded structure.
Acknowledgments
We thank J. M. Herrmann for help with the manuscript and the members of the Schekman Lab for discussion and encouragement. This work was supported by the Howard Hughes Medical Institute (R.S.), the Swiss National Science Foundation (Grant 31-43366.95 to L.O.) and the Human Frontier Science Program Organization (L.O.). A.S. was supported by a European Molecular Biology Organization long term fellowship.
ABBREVIATIONS
- DOPA
dioleoylphoshatidic acid
- DOPC
dioleoylphosphatidylcholine
- DOPE
dioleoylphosphatidylethanolamine
- DOPS
dioleoylphosphatidylserine
- NBD-
7-(nitrobenz 2-oxa-1,3 diazol-4yl)-
- NBD-PE
1-oleoyl-2-NBD-N-aminododecanoyl-sn-glycero-3-phosphoethanolamine
- PA
phosphatidic acid
- PIP
phosphatidylinositol 4-phosphate
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PLD
phospholipase D
- GTP-γ-S
guanosine 5′-[γ-thio]triphosphate
- ER
endoplasmic reticulum
- PI
phosphatidylinositol
- POPC/POPE
1-palmitoyl-2-oleoyl-phosphatidyl choline/1-palmitoyl-2-oleoyl-phosphatidylethanolamine
- PS
phosphatidylserine
- GDP-β-S
guanosine 5′-[β-thio] diphosphate
References
- 1.Robinson M S. Curr Opin Cell Biol. 1994;6:538–644. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 2.Bednarek S Y, Orci L, Schekman R. Trends Cell Biol. 1996;6:468–473. doi: 10.1016/0962-8924(96)84943-9. [DOI] [PubMed] [Google Scholar]
- 3.Schekman R, Orci L. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 4.Bednarek S, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 5.Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Söllner T H, Rothman J E. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 6.Salama N R, Schekman R W. Curr Opin Cell Biol. 1995;7:536–543. doi: 10.1016/0955-0674(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 7.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 8.Schekman R, Mellman I. Cell. 1997;90:197–200. doi: 10.1016/s0092-8674(00)80326-8. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson J G, Finazzi D, Klausner R D. Nature (London) 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 10.Helms J B, Rothman J E. Nature (London) 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson J G, Cassel D, Kahn R A, Klausner R D. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini T, Orci L, Amherdt M, Brunner M, Kahn R A, Rothman J E. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- 13.Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman J E. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- 14.Ktistakis N T, Brown H A, Waters M G, Sternweis P C, Roth M G. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuehn M J, Schekman R. Curr Opin Cell Biol. 1997;9:477–483. doi: 10.1016/s0955-0674(97)80022-1. [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka K, Orci L, Amherdt M, Bednarek S Y, Hamamoto S, Schekman R, Yeung T. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 17.Kahn R A, Randazzo P, Serafini T, Weiss O, Rulka C, Clark J, Amherdt M, Roller P, Orci L, Rothman J E. J Biol Chem. 1992;267:13039–13046. [PubMed] [Google Scholar]
- 18.Hosobuchi M, Kreis T, Schekman R. Nature (London) 1992;360:603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- 19.Zinser E, Daum G. Yeast. 1995;11:493–536. doi: 10.1002/yea.320110602. [DOI] [PubMed] [Google Scholar]
- 20.Randazzo P A, Weiss O, Kahn R A. Methods Enzymol. 1992;219:362–369. doi: 10.1016/0076-6879(92)19036-6. [DOI] [PubMed] [Google Scholar]
- 21.Orci L, Palmer D J, Ravazzola M, Perrelet A, Amherdt M, Rothman J E. Nature (London) 1993;362:648–652. doi: 10.1038/362648a0. [DOI] [PubMed] [Google Scholar]
- 22.Orci L, Palmer D, Amherdt M, Rothman J E. Nature (London) 1993;364:732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- 23.Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 24.Letourneur F, Gaynor E C, Hennecke S, Demoullière C, Duden R, Emr S D, Riezman H, Cosson P. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 25.Aoe T, Cukierman E, Lee A, Cassel D, Peters P J, Hsu V W. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Springer S H, Schekman R. Science. 1998;281:698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- 27.Barlowe C, Schekman R. Nature (London) 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 28.Peyroche A, Paris S, Jackson C L. Nature (London) 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- 29.Hammond S M, Altshuller Y M, Sung T C, Rudge S A, Rose K, Engebrecht J, Morris A J, Frohman M A. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- 30.Waksman M, Eli Y, Liscovitch M, Gerst J E. J Biol Chem. 1996;271:2361–2364. doi: 10.1074/jbc.271.5.2361. [DOI] [PubMed] [Google Scholar]