Abstract
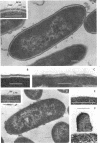
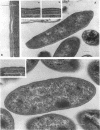
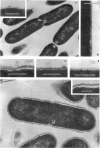
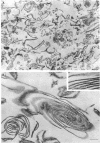
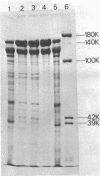
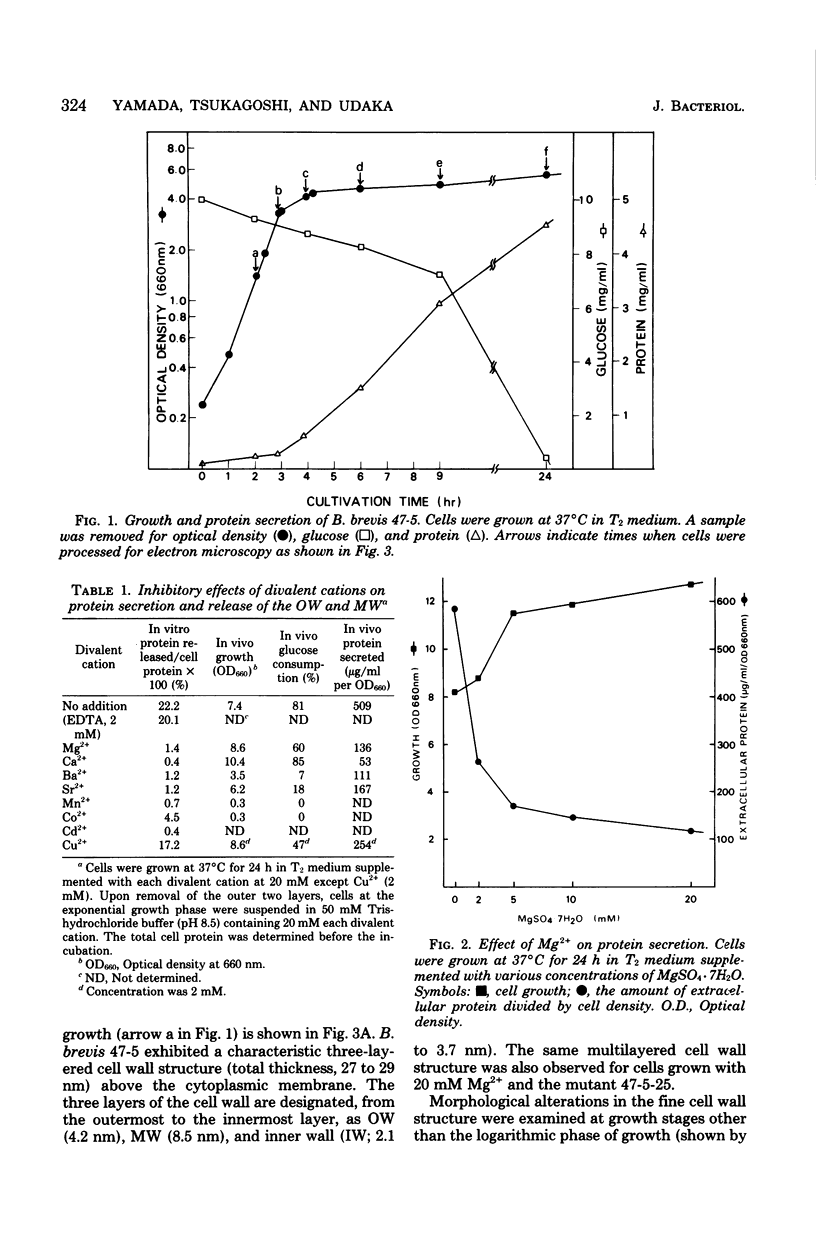
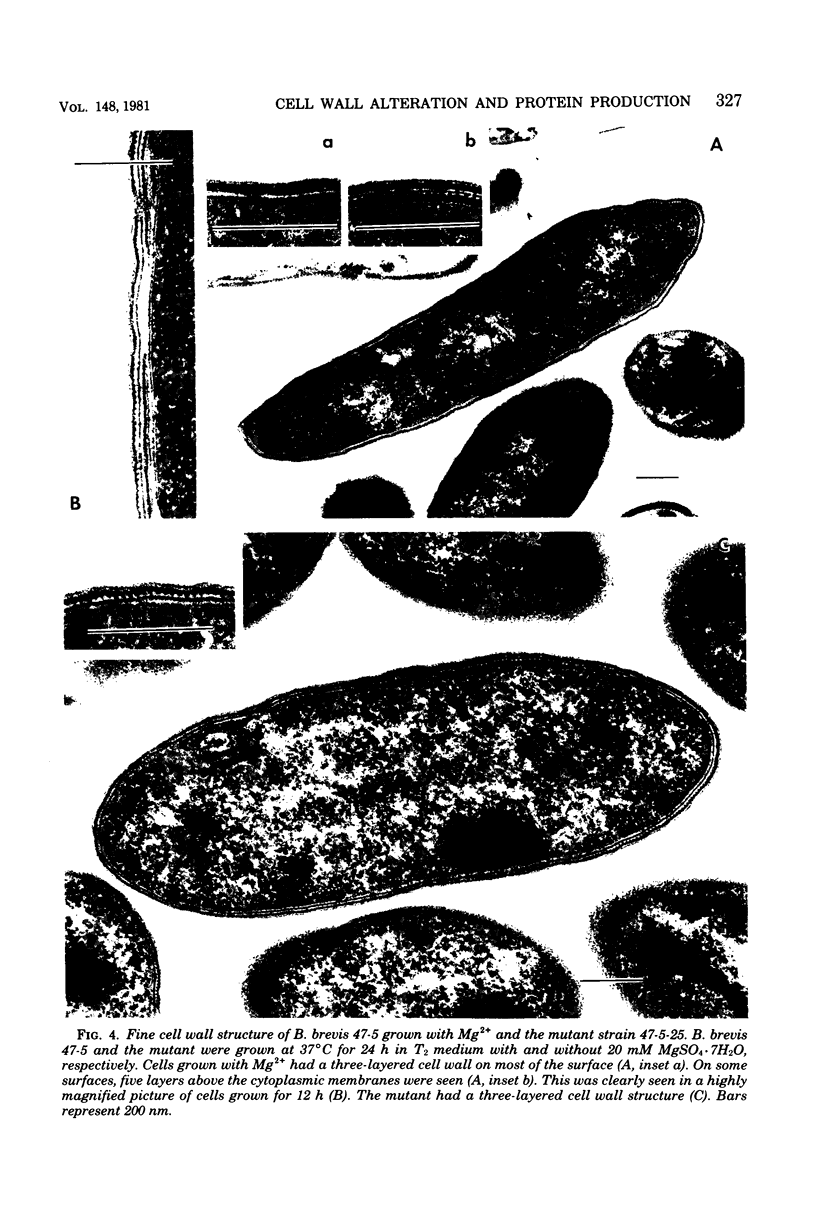
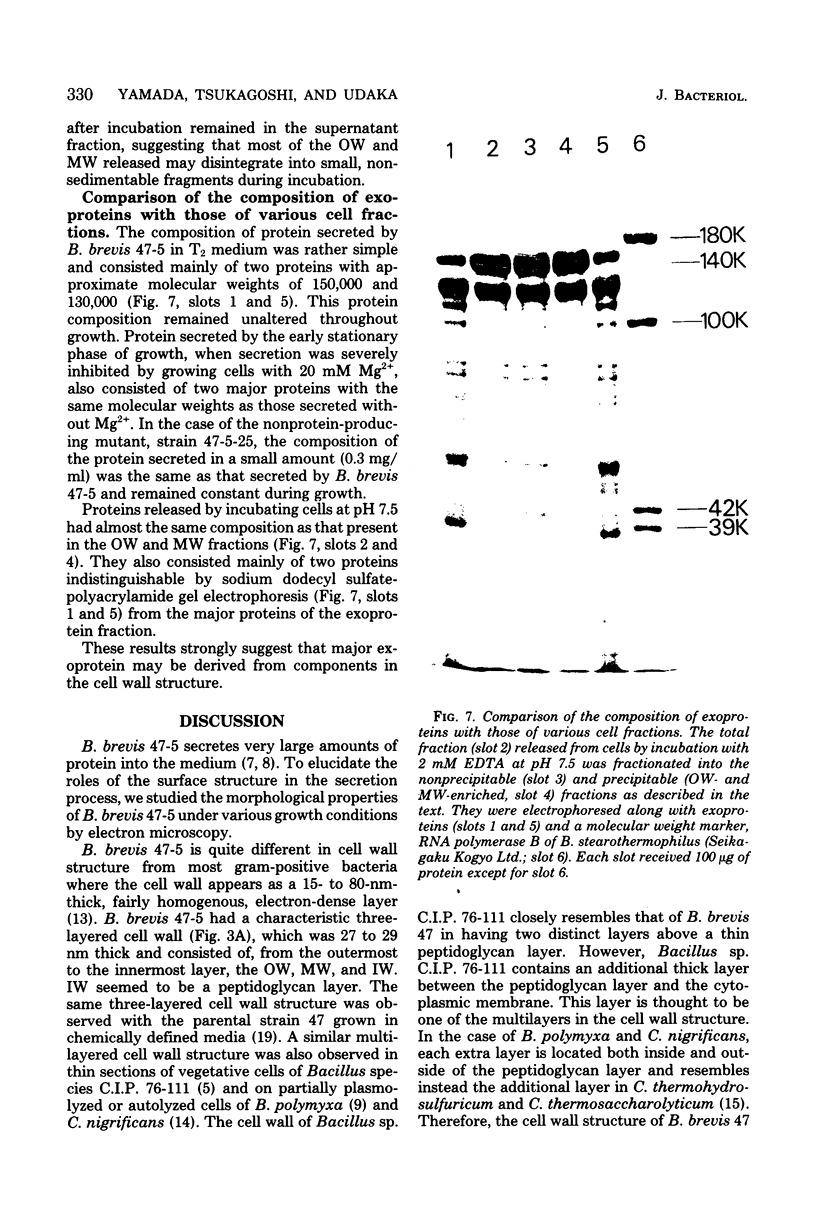
Bacillus brevis 47 secreted vast amounts of protein into the medium and had a characteristic three-layered cell wall. The three layers are designated, from the outermost to the innermost layer, as the outer wall (4.2 nm), the middle wall(8.5 nm), and the inner wall (2.1-3.7 nm). The inner wall might be a peptidoglycan layer. The fine cell wall structure was morphologically altered to various extents, depending on the growth period. At the early stationary phase of growth, cells began to shed the outer two layers of a limited area of the surface. This shedding was complete after further cell growth. The morphological alterations in the cell wall occurred concomitantly with a prominent increase in protein excretion. When protein secretion was severely inhibited by growing cells with Mg2+, morphological alterations in the cell wall were not observed, even at the late stationary phase of growth. This was also the case with a nonprotein-producing mutant, strain 47-5-25. When cells were incubated in buffers, the outer two layers of the cell wall were specifically removed, leaving cells surrounded only by the inner wall layer. The layers removed by incubation were recovered by high-speed centrifugation. This fraction consisted of two layers resembling the outer and middle wall layers. Protein secreted by B. brevis 47-5 consisted mainly of two proteins with approximate molecular weights of 150,000 and 130,000. Proteins released by incubating cells in buffers and proteins in the outer- and middle-wall-enriched fraction were also composed mainly of two proteins with the same molecular weights as those secreted into the medium. Therefore, we conclude that B. brevis 47 secretes proteins derived from the outer two layers of cell wall and these components are synthesized even after the shedding of the outer two layers.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J., Murray R. G. Surface arrays on the cell wall of Spirillum metamorphum. J Bacteriol. 1975 Dec;124(3):1529–1544. doi: 10.1128/jb.124.3.1529-1544.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leduc M., Rousseau M., van Heijenoort J. Structure of the cell wall of Bacillus species C.I.P. 76-111. Eur J Biochem. 1977 Oct 17;80(1):153–163. doi: 10.1111/j.1432-1033.1977.tb11867.x. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E. L'inclusion au polyester pour l'ultramicrotomie. J Ultrastruct Res. 1958 Dec;2(2):200–214. doi: 10.1016/s0022-5320(58)90018-2. [DOI] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Glauert A. M. Ultrastructure of the cell walls of two closely related clostridia that possess different regular arrays of surface subunits. J Bacteriol. 1976 May;126(2):869–882. doi: 10.1128/jb.126.2.869-882.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U., Adam H., Klaushofer H. Die Feinstruktur der Zellwandoberfläche von zwei thermophilen Clostridienarten, dargestellt mit Hilfe der Gefrierätztechnik. Mikroskopie. 1968 Aug;23(1):1–10. [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Glauert A. M. Synthesis and turnover of the regularly arranged surface protein of Acinetobacter sp. relative to the other components of the cell envelope. J Bacteriol. 1976 Jul;127(1):440–450. doi: 10.1128/jb.127.1.440-450.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi N., Yamada H., Tsuboi A., Udaka S. Effects of Phosphate in Medium on Protein Secretion in a Protein-Producing Bacterium, Bacillus brevis 47. Appl Environ Microbiol. 1981 Aug;42(2):370–374. doi: 10.1128/aem.42.2.370-374.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]