Abstract
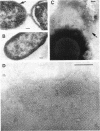
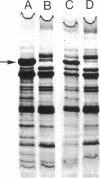
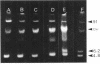
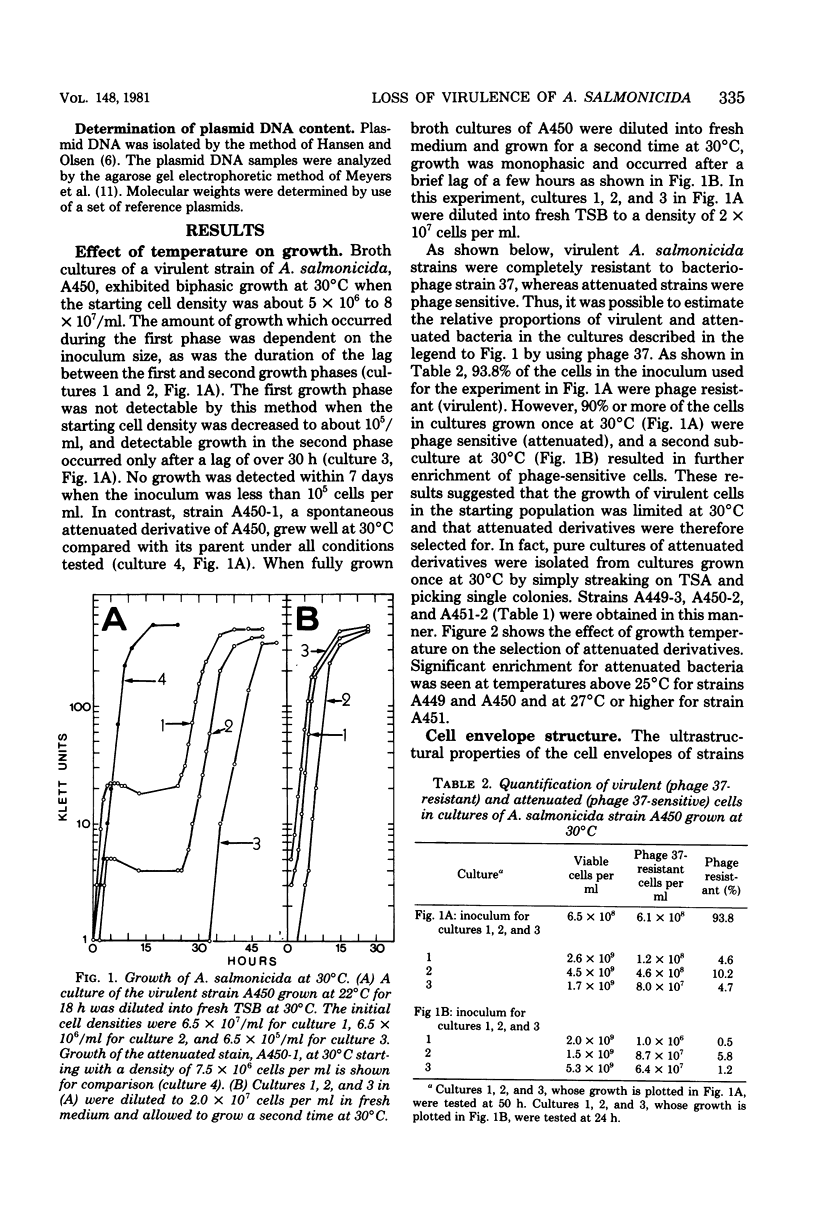
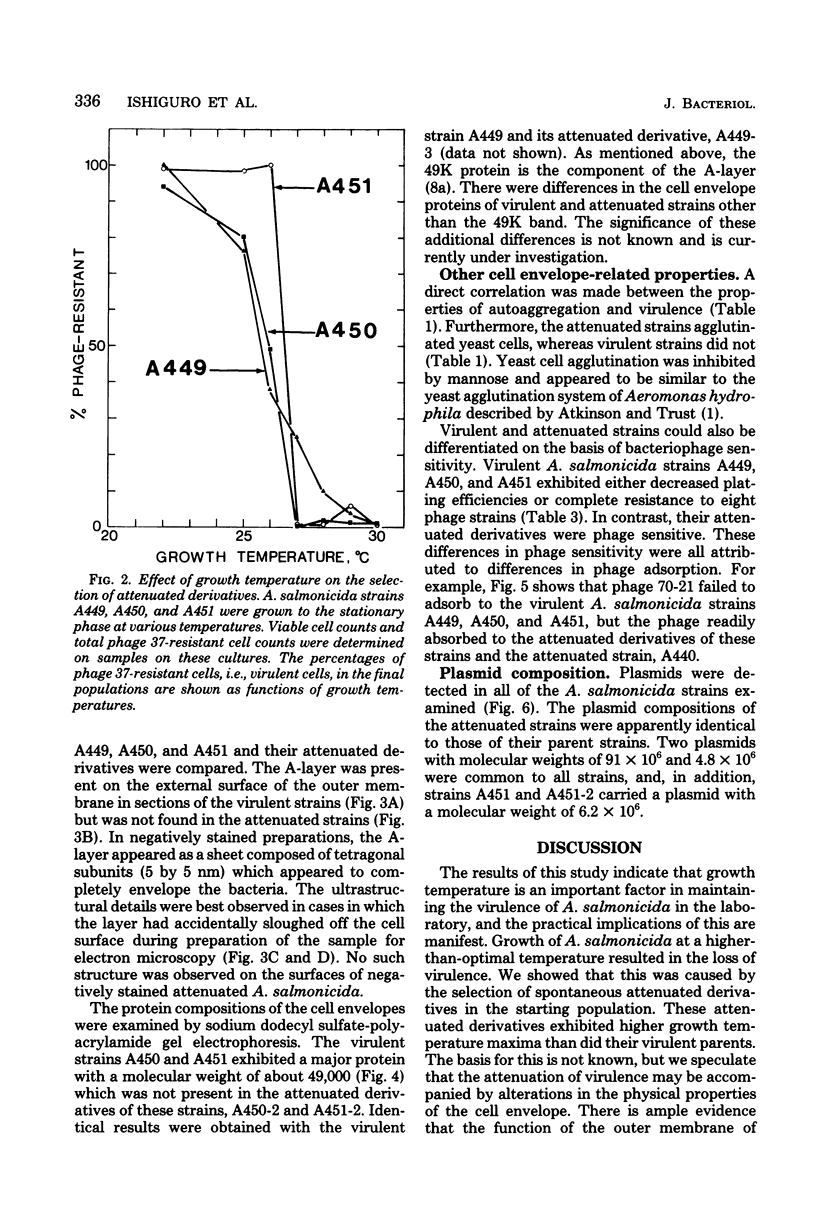
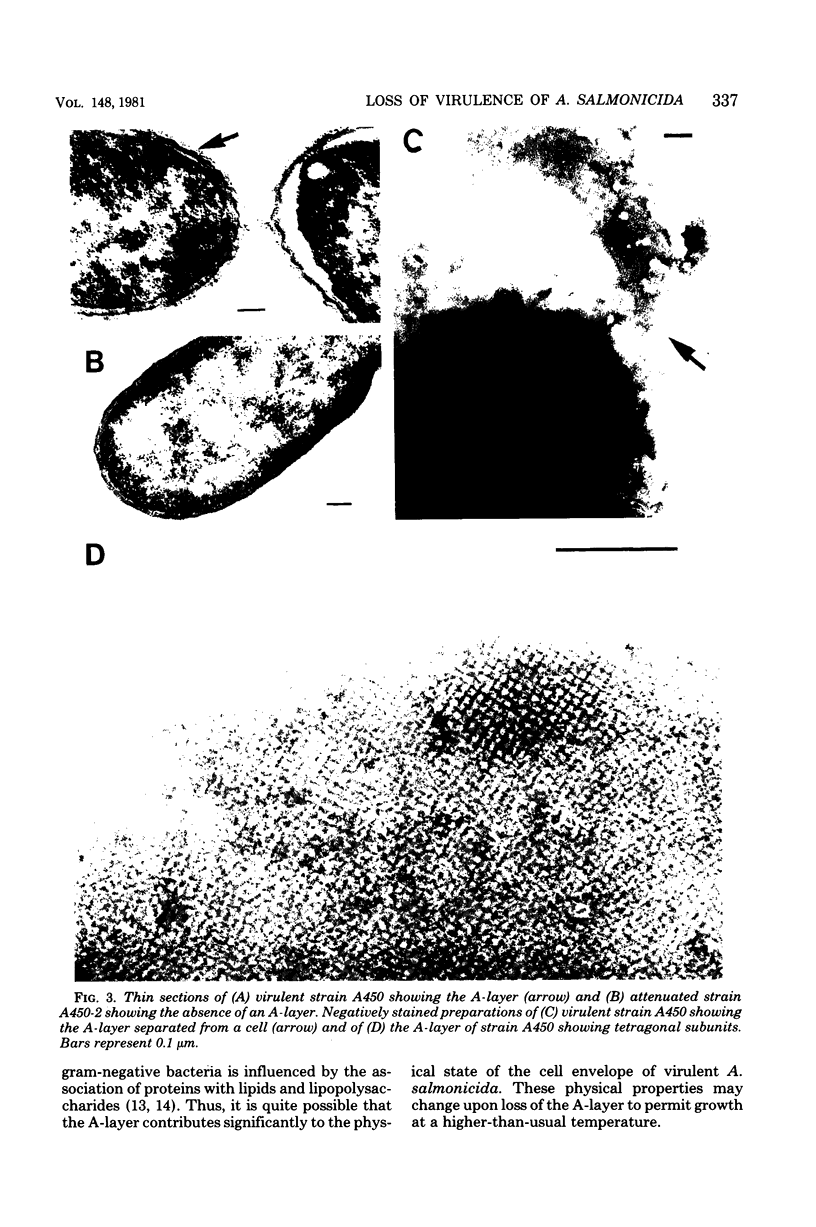
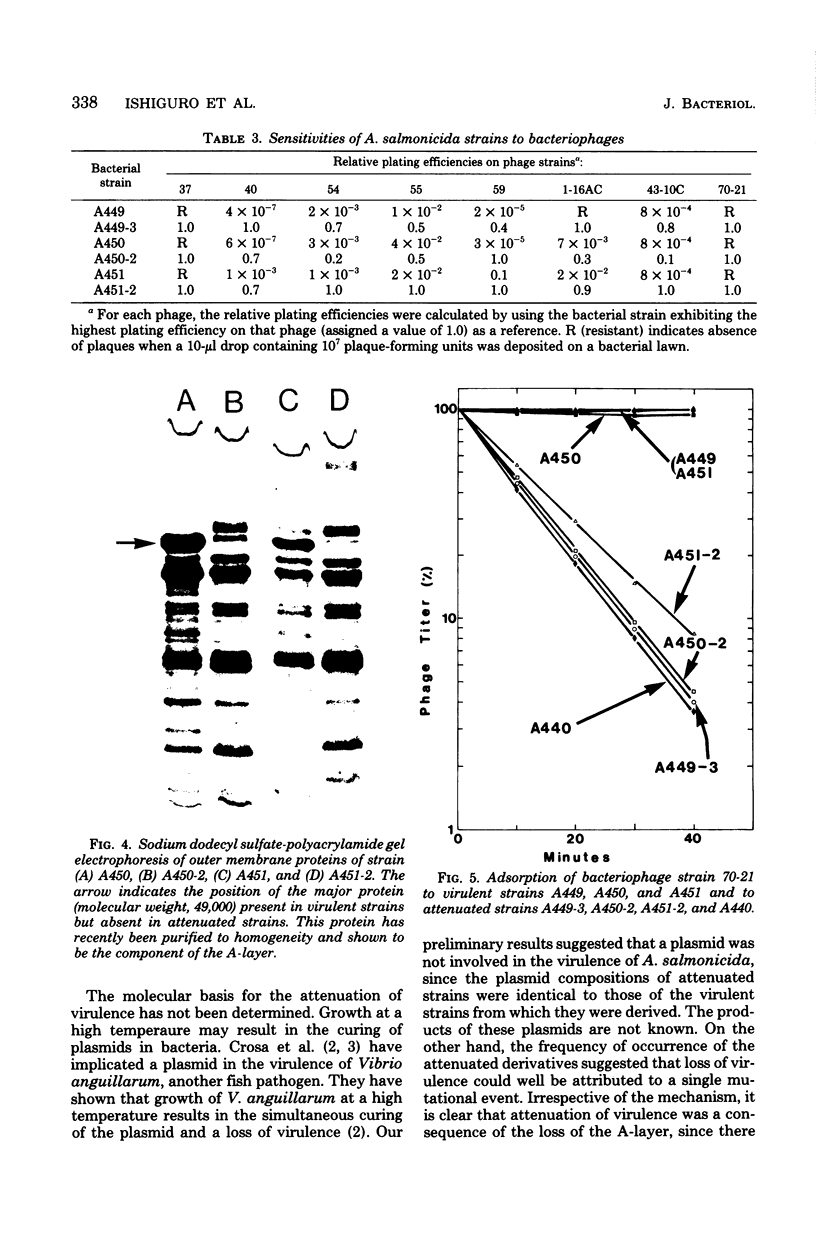
The effect of growth temperature on the loss of virulence of the fish pathogen Aeromonas salmonicida was investigated. Three virulent strains were grown in Trypticase soy broth at temperatures ranging from 22 to 30 degrees C. Growth at a higher-than-optimal temperature (26 to 27 degrees C for the three strains studied) resulted in the selection of spontaneous attenuated derivatives in the initial bacterial population. For example, virulent bacteria represented less than 10% of the population of a culture grown at 30 degrees C, and attenuated derivatives were easily isolated by streaking the culture on solid medium and picking single colonies. Virulent strains autoaggregated during growth and possessed a cell wall layer (A-layer) external to the outer membrane, as previously described. Attenuated strains did not autoaggregate and did not possess the A-layer. The A-layer apparently shielded bacteriophage receptors and a mannose-specific yeast agglutinin located in the outer membrane. Thus, virulent strains exhibited impaired adsorption of phages, whereas attenuated strains were phage sensitive. Furthermore, attenuated strains agglutinated yeast cells but virulent strains did not. The attenuated strains had higher maximum growth temperatures than their virulent parent strains, and this accounts for their selection at high temperatures. It is proposed that the A-layer contributes significantly to the physical properties of the A. salmonicida cell envelope and that these physical properties of the A. salmonicida cell envelope and that these physical change upon loss of the A-layer to permit growth at a higher-than-usual temperature.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson H. M., Trust T. J. Hemagglutination properties and adherence ability of Aeromonas hydrophila. Infect Immun. 1980 Mar;27(3):938–946. doi: 10.1128/iai.27.3.938-946.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L., Schiewe M. H. Curing of a plasmid is correlated with an attenuation of virulence in the marine fish pathogen Vibrio anguillarum. Infect Immun. 1980 Mar;27(3):897–902. doi: 10.1128/iai.27.3.897-902.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Schiewe M. H., Falkow S. Evidence for plasmid contribution to the virulence of fish pathogen Vibrio anguillarum. Infect Immun. 1977 Nov;18(2):509–513. doi: 10.1128/iai.18.2.509-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Buckley J. T., Ishiguro E. E., Phipps B. M., Monette J. P., Trust T. J. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida. J Bacteriol. 1981 Sep;147(3):1077–1084. doi: 10.1128/jb.147.3.1077-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]