Abstract
Two major characteristics of baculovirus infection are arrest of the host cell at G2/M phase of the cell cycle with continuing viral DNA replication. We show that Autographa californica nucleopolyhedrovirus (AcMNPV) encodes for a multifunctional cyclin that may partially explain the molecular basis of these important characteristics of AcMNPV (baculovirus) infection. Amino acids 80–110 of the viral structural protein ODV-EC27 (−EC27) demonstrate 25–30% similarity with cellular cyclins within the cyclin box. Immunoprecipitation results using antibodies to −EC27 show that −EC27 can associate with either cdc2 or cdk6 resulting in active kinase complexes that can phosphorylate histone H1 and retinoblastoma protein in vitro. The cdk6-EC27 complex also associates with proliferating cell nuclear antigen (PCNA) and we demonstrate that PCNA is a structural protein of both the budded virus and the occlusion-derived virus. These results suggest that −EC27 can function as a multifunctional cyclin: when associated with cdc2, it exhibits cyclin B-like activity; when associated with cdk6, the complex possesses cyclin D-like activity and binds PCNA. The possible roles of such a multifunctional cyclin during the life cycle of baculovirus are discussed, along with potential implications relative to the expression of functionally authentic recombinant proteins by using baculovirus-infected cells.
Autographa californica nucleopolyhedrovirus (AcMNPV) infection produces two types of viral progeny. The occlusion-derived viral form (ODV) is optimal for infection of the insect gut, and therefore transmits infection from insect to insect in nature. The budded virus form (BV) transmits infection from cell to cell during secondary infection. ODV infects gut cells by fusion of the viral envelope with the columnar cell microvillar membranes, whereas BV entry of other cells occurs by adsorptive endocytosis. After viral uptake, the nucleocapsid induces actin polymerization (in vitro) that suggests that actin cables may be involved in the movement of nucleocapsids to the nucleus where uncoating occurs (1). ODV and BV differ significantly in their ability to infect the insect through the gut and, viral envelopes and nucleocapsids of the two viral forms contain different proteins, or proteins that are processed differently (2).
Our studies emphasize investigation of the process of ODV envelope maturation that occurs by the abundant proliferation of intranuclear membranes that serve as precursor membranes for ODV envelopes. We note similarities between the phenotype of enlarged nuclei, intranuclear membrane proliferation, and nuclear envelopment during baculovirus infection and the early stages of mitosis in insects (3). In some insect cells, the nuclear envelope may be a source of intranuclear membranes, including small vesicles and an envelope of the spindle apparatus (4–6). However, in AcMNPV-infected cells, the nuclear envelope remains largely intact, with small regional perturbations. Our data support the hypothesis that proliferation of the inner nuclear membrane or nuclear envelope produces the intranuclear membranes essential for ODV nucleocapsid envelopment.
In higher eukaryotes, the entry into mitosis is controlled by activation of the cyclin B-cdc2 complex that results in phosphorylation and disorganization of the nuclear lamina, and increased nuclear envelope fluidity (7–10). By mid-metaphase, the cyclin B-cdc2 complex is inactivated by ubiquinitation and proteolysis of the cyclin B subunit. This allows nuclear envelope dispersal as well as exit from mitosis (9, 11). When cells are transfected with a nondestructible analog of cyclin B, progression through the cell cycle is arrested at M phase with an enlarged nucleus and an intact nuclear envelope (8, 10, 12). We show that AcMNPV infection of Sf9 cells results in host cell cycle arrest at G2/M phase with an intact nuclear envelope, concomitant with sustained cdc2-associated histone H1 kinase activity even after degradation of cellular cyclin B. This data suggests a model where host cell cycle arrest is maintained by an active, viral-encoded nondegradable analog of cyclin B-cdc2 complex. We also note that baculovirus DNA replication still occurs in G2/M phase-arrested cells (3). Thus, during infection, the virus must encode some mechanism to override cellular checkpoints and maintain an “S phase-like” environment conducive for viral DNA replication in G2/M phase-arrested cells.
We now report that AcMNPV encodes a protein (ODV-EC27; −EC27) capable of acting as a multifunctional cyclin. This protein is detected in purified ODV; however, potentially modified forms of this protein are also present in BV (13). −EC27 is present in complexes containing either cdc2 or cdk6 and these complexes can phosphorylate both histone H1 and retinoblastoma protein (pRb) in vitro. Proliferating cell nuclear antigen (PCNA) can also associate with the cdk6-EC27 complex. Additionally, we show that −EC27 and PCNA are both structural proteins of ODV and that PCNA is also present in purified BV. The potential role and function of −EC27 acting like B-type and/or D-type cyclin during the baculovirus life cycle are considered.
MATERIALS AND METHODS
Insect Cell Lines and VirusSpodoptera frugiperda IPLB-Sf21-AE clonal isolate 9 (Sf9) cells were cultured in suspension at 27°C in TNMFH medium supplemented with 10% fetal bovine serum.
Cells were maintained and infected in log phase growth and cell density was ≈1.2–1.5 × 106 cells/ml at the time of infection. Cells were infected at a multiplicity of infection of 20 by using the baculovirus AcMNPV (E2 strain).
Immunoprecipitation and in Vitro Kinase Assays.
Immunoprecipitation with antisera to −EC27 and subsequent kinase assays were performed as described (3, 14). Briefly, Sf9 cells were infected, time course samples were collected and then analyzed for protein content (15). Whole-cell lysate (150 μg total protein) from each sample was dissolved in RIPA buffer 50 mM Tris/150 mM NaCl/0.5% Triton X-100/0.5% deoxycholate, pH 7.5, containing protease and phosphatase inhibitors, precleared by incubating with protein A/G agarose beads and immunoprecipitated with 10 μl of antiserum to −EC27 (no. 7351). Immune complexes were collected on protein A/G agarose beads; washed three times with RIPA buffer and three times in kinase assay buffer (50 mM Tris/10 mM MgCl2/1 mM DTT, pH 7.5). Kinase reactions were initiated by resuspending the −EC27 immunoprecipitate in 20 μl of a reaction mixture consisting of kinase assay buffer (30°C) containing 1 μg histone H1 protein (Boehringer Mannheim) or 500 ng of pRb (Santa Cruz Biotechnology) and 100 μM ATP and 50 μCi of γ-32P-ATP (3,000 Ci/μmol;1 Ci = 37 GBq). Reactions proceeded for 30 min (30°C) and were terminated by addition of 10 μl of 4× SDS sample buffer. The mixture was boiled for 3 min, centrifuged at 16,000 × g (3 min) and the entire sample loaded onto a 15% SDS/PAGE gel. Phosphorylated histone H1 and pRb bands were quantified by PhosphorImager SF (Molecular Dynamics). To confirm equal loading of samples, the gels were stained with Coomassie blue.
Lysates were depleted for individual cdks by three consecutive treatments with 20 μg of antibody specific for the cdk. All antibodies were rabbit polyclonal antibodies (Santa Cruz Biotechnology) and were shown to cross-react with insect cdks (data not shown). Depleted lysates were subsequently treated with antiserum to −EC27 (as above), and the immunoprecipitated samples were assayed for kinase activity by using histone H1 or pRb as substrates.
Western Blot Analysis.
Proteins were separated by using SDS/PAGE (16) and transferred to poly(vinylidene difluoride) membranes by using Western blot techniques (17). The membranes were blocked with tris buffered saline (0.5% Tween 20; 1% dry milk) and primary antibody bound overnight (4°C). Blots were washed and horseradish peroxidase-linked IgG (1:5,000) was bound for 1 h (27°C). Blots were washed and reacted for 1 min with enhanced chemiluminescence reagent (Amersham) and exposed to x-ray film. The fluorescent signal was quantitated by using a ChemiImager (Alpha Innotech, San Leandro, CA).
Immunoelectron Microscopy.
Samples were prepared for electron microscopy using LR White as described by Hong et al. (18). Sections were blocked with tris buffered saline (0.5% Tween 20; 1% BSA) for 15 min, reacted with anti-PCNA (1:200, 16 h, 4°C; Santa Cruz Biotechnology) and washed. The bound antibody was detected by using gold-conjugated anti-rabbit IgG (25 nm; Electron Microscopy Sciences, Ft. Washington, PA). Sections were stained with uranyl acetate (19) and lead citrate (20) and visualized by using a Zeiss 10C transmission electron microscope (Texas A&M University Electron Microscopy Center).
Purification of Virus.
BV, ODV, and envelope and nucleocapsid fractions were purified as described in Braunagel and Summers (2). The purity of the envelope and nucleocapsid fractions was evaluated by using the antisera: BV:gp67, BV/ODV-E26; ODV:ODV-E66, ODV-E56, and BV/ODV-E26.
RESULTS
−EC27 Amino Acids Have Similarity with Cyclins and −EC27 Is Part of a Complex Capable of Phosphorylating Histone H1 and pRb in Vitro.
The cyclin box is a highly conserved feature among cellular and viral cyclins. −EC27 shows an 18% overall similarity to cyclin B, while the region between amino acids 80 and 110 shows 25–30% similarity to the cyclin box of various cyclins (Fig. 1). Outside of the cyclin box, cyclins also contain a conserved site for ubiquinitation (“destruction box”; ref. 12) that is necessary for cyclin B degradation and exit from mitosis. −EC27 does not contain such a destruction sequence (13).
Figure 1.
Comparison of the cyclin box of −EC27 (amino acids 80–110) with the cyclin box of cyclins. Shaded areas represent conservative amino acids compared against the −EC27 sequence. Rules for assignment of conservative amino acids are: G=A=S=T; V=L=I=M=F=Y=W; n = Q=D=E; r = K=H .
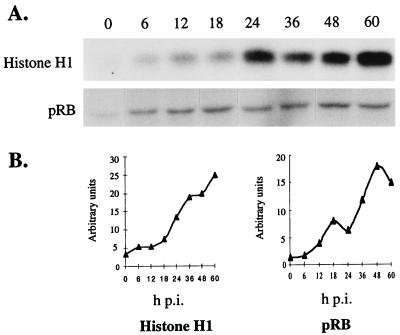
To determine whether −EC27 was part of an active kinase complex, at the designated times postinfection (p.i.), Sf9 cell lysates were immunoprecipitated by using −EC27 antiserum, and kinase activity of the precipitated complexes assayed by using either histone H1 or pRb as in vitro substrate. Quantitative analysis showed that the level of −EC27-associated histone H1 and pRb kinase activity increased by 18 h p.i. reaching maximal levels of activity by 60 h p.i. (Fig. 2 A and B). While pRb phosphorylation decreased slightly between 48–60 h p.i., repetitions of these experiments showed that the decrease was not significant. −EC27 transcripts were detected by 16 h p.i. and protein detected by 18 h p.i.; thus the increase in kinase activity was similar to the temporal expression of −EC27 (Fig. 5A; ref. 13). We also noted that −EC27 immunoprecipitated complexes from cell lysates between 6–18 h p.i. have a low level of pRb-associated kinase activity. It may be that −EC27 antibody cross-reacts with a cellular protein, or some activity is provided by input virus, but we have no definitive explanation for this activity.
Figure 2.
−EC27-associated kinase activity. (A) Immunoprecipitation using antibody to −EC27 was performed from infected Sf9 cell lysates at indicated times p.i. The kinase activity of the precipitated complex was then assayed by using either histone H1 or pRb as the in vitro substrate. (B) Phosphorylated histone H1 or pRb bands were quantitated by using a PhosphorImager SF (Molecular Dynamics).
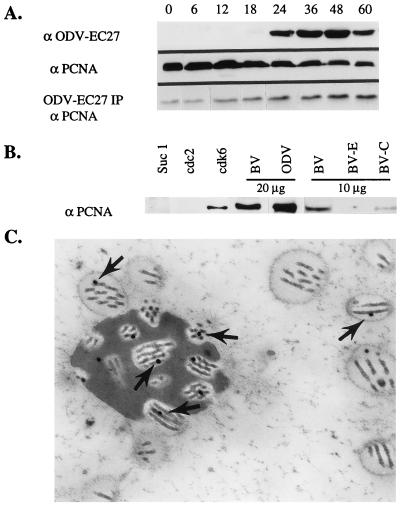
Figure 5.
Association of PCNA with Cdk6–EC27 complex and AcMNPV nucleocapsid. (A) Twenty-five micrograms of total protein from infected Sf9 cell lysates (h p.i.) or −EC27 immunoprecipitated complex (ODV-EC27 IP) from 150 μg of whole-cell lysate (h p.i.) were separated by using SDS/PAGE and analyzed by using Western blot and antibodies to either −EC27 (α-ODV-EC27) or PCNA (α-PCNA). (B) Antibodies to Suc1, cdc2, and cdk6 were used to immunoprecipitate the protein complex from Sf9 cell lysate (60 h p.i.). The proteins were separated on SDS/PAGE gels and analyzed by Western blot by using PCNA antibodies. Proteins of purified BV and ODV (20 μg) were separated by using SDS/PAGE and analyzed by Western blot by using PCNA antibodies. BV was fractionated into envelope (BV-E) and nucleocapsid (BV-C) and preparations (10 μg/sample) were probed by using PCNA antibodies (α-PCNA). (C) ImmunoGold localization of PCNA to nucleocapsid of ODV (arrows).
−EC27 Associates with Cdc2 and Cdk6.
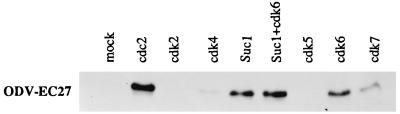
To identify the serine/threonine kinase associated with −EC27, we tested selected cdks that are known to associate with cellular and viral cyclins and form active kinase complexes (21). Infected cell extracts (60 h p.i.) were incubated with antibodies to selected cdks. Immune complexes were collected on protein A/G agarose beads, separated by SDS/PAGE, and Western blot analysis performed by using antiserum to −EC27. −EC27 coprecipitated with cdc2, suc1, cdk6 and to a lesser extent, cdk7 (Fig. 3).
Figure 3.
Identification of protein kinases interacting with −EC27. Sf9 cell lysates were prepared at 60 h p.i. and used for immunoprecipitation with antibodies to cdc2, cdk2, -4, -5, -6, -7, and Suc1. Immunoprecipitated complexes were separated by SDS/PAGE and Western blot performed by using antisera to −EC27.
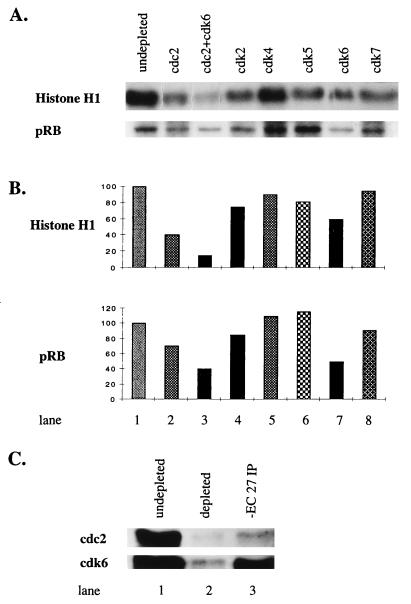
To determine which of the cdk’s subunits were responsible for kinase activity, lysates were prepared from infected cells (60 h p.i.) and immunodepleted for the individual kinase. Immunodepletion removed ≈70–80% of the cdk proteins (Fig. 4C, lanes 1 and 2; and data not shown). Kinase activity of the −EC27 immunoprecipitated complexes from these depleted lysates was determined. −EC27-associated histone H1 kinase activity was reduced 50–60% when cell lysates were depleted of cdc2 and reduced 30–40% when cdk6 was depleted (Fig. 4 A and B, lanes 2 and 7). When both cdc2 and cdk6 were depleted, kinase activity decreased 85–90% (Fig. 4 A and B, lane 3). The removal of cdk2, cdk5, or cdk7 resulted in a 15–20% decrease in activity, while depletion of cdk4 showed no significant change in kinase activity (Fig. 4 A and B, lanes 4–6, 8). Using pRb as in vitro substrate, depletion of cdk6 decreased −EC27-associated kinase activity ≈50%, while depletion of cdc2 reduced activity by 25% (Fig. 4B, lanes 2, 7). When both cdc2 and cdk6 were depleted, −EC27-associated pRb kinase activity was reduced by 55% (Fig. 4B, lanes 4–6, 8). The residual activity of pRB phosphorylation after removal of cdc2 and cdk6, could be caused by the presence of other, unidentified protein(s) capable of interacting with −EC27 to form an active kinase. Removal of cdk7 caused minimal effects. Depletion of cdk4 and cdk5 antibodies resulted in a 20–30% increase of −EC27-associated pRb kinase activity (Fig. 4B; lanes 4–6, 8). It is possible depletion of cdk4 and cdk5 resulted in removal of putative inhibitor(s) of −EC27-associated kinase activity. Finally, −EC27 immunoprecipitated complexes were separated by SDS/PAGE, and Western blot analyses showed that the precipitated complex contained cdc2 and cdk6 (Fig. 4C; lane 3). These data indicate that the interaction with cdc2 and cdk6 can largely account for the observed −EC27-associated kinase activity.
Figure 4.
−EC27 Association with Cdc2 and Cdk6 results in kinase activity. (A) At 60 h p. i. Sf9 cell lysates were immunodepleted using antibodies to indicated cdks. −EC27 immunoprecipitated complexes from these depleted lysates were obtained and then tested for kinase activity by using either histone H1 or pRb as in vitro substrate. Samples were separated by using SDS/PAGE, and phosphorylated bands of histone H1 and pRb are shown. (B) Phosphorylated histone H1 and pRb bands were quantitated by using a PhosphorImager SF (Molecular Dynamics). These data were normalized, assigning the levels of phosphorylation in undepleted lysates as 100%. (C) Lanes 1–2: Comparing the levels of cdc2 and cdk6 in Sf9 cell lysates before and after immunodepletion with cdc2 and cdk6 antibodies respectively. Lane 3: At 60 h p.i. cell lysate was immunoprecipitated by using −EC27 antibody and analyzed by Western blot by using antibody to either cdc2 or cdk6.
PCNA Associates with −EC27 and AcMNPV Nucleocapsids.
The data suggest that −EC27 forms a functional complex with cdk6, the catalytic subunit normally activated by D-type cyclins. Cdk6-cyclin D complexes regulate cell cycle transition from G1 to S phase and can bind PCNA. We speculated that the cdk6-EC27 complex might also associate with PCNA. As controls, cell lysates from various times p.i. were separated by SDS/PAGE, and analyzed by Western blot analysis using antibodies to −EC27 or PCNA. −EC27 was detected at 18 h p.i., increased to higher levels by 24 h p.i. and remained at high, steady-state levels throughout infection (Fig. 5A; α ODV-EC27). PCNA was present in uninfected cell lysates (time 0) and the total amount of PCNA decreased as infection progressed (Fig. 5A; α PCNA). To determine whether PCNA associated with complexes containing −EC27, complexes were immunoprecipitated using antibody to −EC27, separated by using SDS/PAGE and analyzed by Western blot using antibodies to PCNA. The amount of PCNA detected in the −EC27 immunoprecipitates increased as infection progressed and roughly paralleled the increase in amount of −EC27 (Fig. 5A; ODV-EC27 IP; α PCNA). There were always detectable amounts of PCNA in −EC27 immunoprecipitated samples from uninfected cells and early times p.i. It is possible that −EC27 antibodies cross-react with a cellular protein that also binds PCNA and this interaction could also account for the low levels of pRb phosphorylation seen at early times p.i. (Fig. 2). AcMNPV codes for a protein with homology to PCNA (22) however, we do not know whether the cellular PCNA antibody cross reacts with viral PCNA. Because −EC27 was capable of forming complexes with cdc2 and cdk6 (Fig. 3), we determined which of these complexes was capable of binding PCNA. Cell lysates (60 h p.i.) were immunoprecipitated using antibodies to cdc2, suc1, or cdk6. The proteins were separated by SDS/PAGE and analyzed by Western blot using antibodies to PCNA (Fig. 5B). PCNA was detected only in the complex containing cdk6 (Fig. 5B).
−EC27 is a structural protein of ODV (13). Association of the cdk6-EC27 complex with PCNA suggested that PCNA could also be a structural protein of the virus. AcMNPV BV and ODV were purified, each separated into envelope (E) and nucleocapsid (C) fractions (2), the proteins separated by SDS/PAGE and analyzed by Western blot using antisera to PCNA. PCNA was present in both BV and ODV and detected only in the nucleocapsid fraction (Fig. 5B and data not shown). The presence of PCNA within the viral nucleocapsid of ODV was confirmed by using electron microscopy and ImmunoGold labeling (Fig. 5C; arrows).
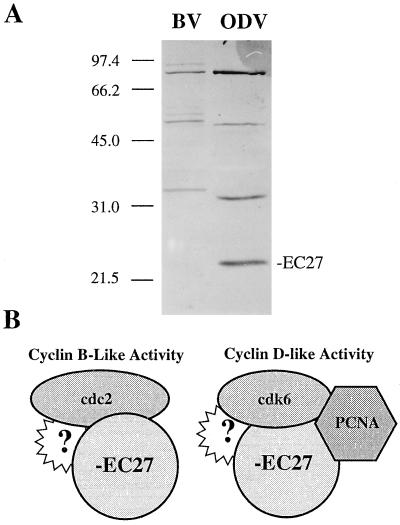
When AcMNPV-infected cell lysates were analyzed by Western blot analysis using −EC27 antibody, only one protein product of 27 kDa was detected during the time course of infection (Fig. 5A; α-ODV-EC27). −EC27 was initially identified as a 27-kDa protein that was specific for ODV, however, we noted that −EC27 antibodies detected several higher molecular mass bands in purified BV and ODV (Fig. 6A; ref. 13). The higher molecular mass proteins (≈37, 61, and 90 kDa) are only detected in purified virus. A series of SDS/PAGE sample buffers (varying concentrations of SDS, DTT, temperature, and times of treatment) were tested and all conditions gave the same results (data not shown). Thus, if these higher molecular weight proteins represent oligomeric forms of −EC27, the oligomers are highly resistant to denaturation. When comparing BV and ODV, the proteins in BV migrate at a slightly higher molecular mass compared with ODV.
Figure 6.
−EC27 immunoreactive proteins of BV and ODV and model of interaction of −EC27 with Cdc2, Cdk6 and PCNA. (A) Purified BV and ODV were separated by using SDS/PAGE and analyzed by Western blot using −EC27 antibodies. The 27-kDa form is unique to ODV (this data is reproduced from ref. 13). (B) −EC27 can associate with either cdc2 (cyclin B-like activity) or cdk6 (cyclin D-like activity). The cdk6−EC27 complex is also capable of binding to PCNA. Protein “?” represents other potential, currently unknown partners.
DISCUSSION
AcMNPV −EC27 is the second viral-encoded cyclin homolog to be reported (23–26). Two herpesviruses (Herpesvirus saimiri and Karposi’s sarcoma-associated herpesvirus) code for a homolog to cyclin D. The herpesvirus cyclin associates with cdk6 (23–25) and catalyzes in vitro phosphorylation of histone H1 and pRb (26). The complex may be resistant to inhibition by the cdk inhibitors, p21Cip1, p27Kip1 and p16Ink-4a. Expression of the herpesvirus cyclin prevents G1 arrest imposed by each inhibitor and stimulates cell cycle progression in quiescent fibroblasts (23, 25). Thus, the herpesvirus cyclin-kinase complexes are able to mediate progression of events through cell cycle checkpoints (26).
The baculovirus cyclin, −EC27 has distinct functional characteristics compared to cellular and viral cyclins. Depending on the cdk protein, and perhaps other viral or cellular proteins yet to be described, the kinase-EC27 complex may have either cyclin B- or D-like activity (Fig. 6B). Thus two models of −EC27 cyclin-like activities can be considered.
Cyclin B-like Function.
Baculovirus infection results in an enlarged cell and nucleus, increased fluidity of an intact nuclear envelope, induction of microvesicles and membranes in the nucleoplasm, and arrest of the host cell in G2/M phase with sustained cdc2-associated histone H1 kinase activity (3). A functional cyclin B-like kinase (cdc2-EC27) could partially explain these major phenotypic characteristics. Cellular cyclin B-cdc2 function is key in the progression of the cell from G2 to M phase. The complex accumulates in the nucleus, is activated by cdc25, and phosphorylates nuclear lamins. Phosphorylation results in breakdown of the nuclear lamina and increased nuclear envelope fluidity. Cyclin B is then degraded and, concomitant with cyclin B destruction, the cell progresses to anaphase with fenestration and/or dispersal of the nuclear envelope. If cyclin B is not degraded, cells are arrested at mid-metaphase with an intact nuclear envelope (8–12). Using antibodies to cyclin B, we previously reported that cellular cyclin B levels accumulate at the time of baculovirus G2/M arrest and are then rapidly degraded (3). This data suggest that the cellular cdc2-cyclin B complex was active and had likely phosphorylated and dispersed the nuclear lamins, thus releasing nucleoskeleton structural constraints on the infected cell nuclear envelope. The identification of a viral cyclin capable of cyclin B-like activity suggests that after the degradation of cellular cyclin B, certain structural and functional dynamics of the nuclear envelope can be maintained by the active cdc2-EC27 complex. Such a model would predict increased fluidity of the nuclear envelope to allow the production of intranuclear microvesicles, which begin to accumulate at the time of G2/M arrest and are precursors to the ODV envelope. However, our previous data showed that formation of protein-rich intranuclear vesicle structures can accumulate within the nucleoplasm even when infected cells are arrested at G1/S phase (3). Thus, it is likely that cdc2-EC27 activity is not the only factor(s) that function to maintain nuclear envelope fluidity for the virus-induced processes responsible for the formation of intranuclear microvesicles and viral envelopes.
The selective advantage(s) for AcMNPV to arrest the host cell cycle at G2/M phase may not be restricted to changes in the dynamics of the nuclear envelope and maturation of ODV. Infection with HIV-1 results in host cell cycle arrest at G2/M phase. At this phase viral gene transcription is optimized at the expense of host (cellular) gene transcription. It was suggested that one advantage of maintaining the host cell cycle in G2/M phase could be to optimize virus production through regulating viral gene transcription (27). Although the Sf9 cell cycle-dependent rate of AcMNPV transcription has not been studied, it was shown that there is a significant reduction in host cell RNA and protein synthesis between 12 and 18 h p.i. (28). This time frame correlates well with the time of AcMNPV induced Sf9 cell cycle arrest at G2/M phase (3).
Cyclin D-like Function.
AcMNPV must have the capacity to influence or regulate cellular vs. viral DNA replication. Using transfection techniques, Prikhod’ko and Miller (29) showed that the viral; ie−2 gene product was sufficient to arrest Sf9 cells in S phase. The arrested cells did not undergo mitosis, had abnormally large nuclei and contained greater than 4N DNA content. In contrast, results from viral infection show cells are arrested at G2/M phase, with no detectable increase in cellular DNA content (3). Thus, during viral infection cellular DNA replication is not detectable, while viral DNA replication occurs at a rapid rate. Surprisingly, even after infected cells were arrested at G2/M phase, viral DNA replication continues that suggest that AcMNPV could code for protein(s) capable of “over-riding” those cell cycle checkpoints. A cdk6-EC27 complex resulting in cyclin D-like activity may partially explain continuing viral DNA replication during G2/M phase arrest. Cyclin D1 associates with four different kinases (cdk2, cdk4, cdk5, and cdk6), PCNA, and the inhibitor p21 (30, 31). The binding and phosphorylation of pRb by cdk-cyclin D promotes the transition from G1 to S phase (32, 33). In addition, cdk-cyclin complexes can “titrate” cdk inhibitors such as kinase inhibitory protein-1 (p27Kip1) into ternary complexes resulting in activation of the cdk2-cyclin E complex (34). Cdk2-cyclin E can then affect components of preinitiation complexes to trigger DNA replication (35). Thus, the presence of an active cdk6-EC27 complex (cyclin D-like activity) could result in maintaining an active replication complex even when cells are outside of S phase. Other viruses (human papillomavirus, adenovirus, and others) depend on the host cell entry into S phase to replicate their viral DNA, and have developed various mechanisms to establish an S phase environment (for review; see ref. 36), and infection with human cytomegalovirus stimulates replication of only viral DNA (37).
PCNA, an auxiliary factor for DNA polymerases β and γ, is essential for eukaryotic DNA replication and repair (38). Mammalian cells contain soluble (free) and insoluble (chromatin-bound) populations of PCNA, and the bound state of PCNA is regulated by the cell cycle (39, 40). The regulated distribution of PCNA from the soluble to insoluble form can function in G2/M arrest presumably by modifying the assembly, stoichiometry and/or subcellular targeting of the cyclin-cdk-p21-PCNA quaternary complex (41, 42, 43). AcMNPV encodes a homolog of PCNA [vPCNA; refs. 22 and 28] and while an obvious function of vPCNA would be as an accessory factor for viral DNA polymerase, a role of vPCNA in viral DNA replication has not been observed (44). Cells infected with a recombinant virus where the C terminus of the vPCNA gene was deleted, exhibited a slight delay in late gene expression (45). PCNA may not be an essential gene for baculoviruses because a PCNA homolog has not been identified in the genome of Bombyx mori NPV (46).
Because both −EC27 and PCNA are structural proteins of ODV and would be presented to the gut cells of the insect immediately upon infection, another function for this protein complex is possible. The columnar cells of the gut are differentiated cells (noncycling) and are a site of primary infection. It may be that the presentation of −EC27, PCNA and perhaps other viral structural proteins, along with the expression of immediate early genes, establishes an “S phase-like” environment in these cells. This environment then facilitates viral DNA replication. PCNA is also detected in purified BV; however, the 27-kDa form of −EC27, which is always associated with active kinase complexes, is not detected. BV contains −EC27 immunoreactive bands at higher molecular masses. This suggests that the −EC27 immunoreactive forms may be differentially processed or stably complexed with itself or other proteins in BV (Fig. 6A; ref. 13). If so, it is possible that these “complexed” forms of −EC27 may also function to promote an S phase-like environment when BV infects secondary tissues, some of which are also differentiated cells (i.e., fat body). In addition to the 27-kDa form, ODV also contains higher molecular mass, immunoreactive forms of −EC27. Thus, the composition and function(s) of the −EC27 complex may be more complicated than the simple model presented here. The process of infection by either BV or ODV clearly can introduce these proteins into host cells and may provide AcMNPV with the ability to redirect the host cellular processes to benefit virus infection.
Other AcMNPV Gene Products that May Function in Cell Cycle Regulation.
While it is not known whether baculovirus-encoded kinases and/or phosphatases function in virus-induced cell cycle arrest, we note that AcMNPV codes for two protein kinases, a protein kinase-interacting protein, and at least one protein phosphatase (47–51). The baculovirus pk-1 gene encodes a serine-threonine kinase catalytic subunit that is capable of phosphorylating histone H1. Pk-1 is first detected at 12 h p.i. and accumulates to high levels during the late phase of infection (50), and protein kinase activity is associated with both BV and ODV (52). It will be of interest to see whether any of these proteins interact with −EC27 and whether they function in virus-induced cell cycle arrest or viral DNA replication.
The results from this study show that AcMNPV encodes a multifunctional cyclin. Depending on its kinase partner −EC27 may either: (i) associate with cdc2 kinase and provide an undegradable analog of cdc2-B cyclin complex or (ii) associate with cdk6 and provide cyclin D-like activity. The significance of the cdk6-EC27-PCNA complex is unknown; however, we note that PCNA is a component of both BV and ODV. The apparent multifunctional roles of −EC27 may include arrest of the host cell cycle at G2/M phase, or regulation of viral and/or cellular DNA replication.
Baculovirus Expression Vector System.
The polyhedrin and p10 gene promoters are widely used for protein expression and are maximally active during the late and very late phases of infection, times when the infected cells are arrested in G2/M phase. Thus, foreign genes expressed under the control of these promoters are expressed in a G2/M phase environment. The additional observation that baculovirus codes for it own unique multifunctional cyclin has special implications for studies where cell cycle regulated products (cyclins, cdks, etc.) are being expressed. These proteins may have modifications unique to baculovirus or G2/M phase arrest.
Acknowledgments
This study was supported in part by National Institutes of Health Grant R01GM47552 (M.D.S. and S.C.B.) and the Texas Agricultural Experiment Station Project TEXO8078 (M.D.S.).
ABBREVIATIONS
- AcMNPV
Autographa californica nucleopolyhedrovirus
- ODV
occlusion-derived virus
- −EC27
ODV-EC27
- BV
budded virus
- p.i.
postinfection
- PCNA
proliferating cell nuclear antigen
- pRb
retinoblastoma protein
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. U04051).
References
- 1.Lanier L M, Volkman L E. Virology. 1998;243:167–177. doi: 10.1006/viro.1998.9065. [DOI] [PubMed] [Google Scholar]
- 2.Braunagel S C, Summers M D. Virology. 1994;202:315–328. doi: 10.1006/viro.1994.1348. [DOI] [PubMed] [Google Scholar]
- 3.Braunagel S C, Parr R, Belyavskyi M, Summers M D. Virology. 1998;244:195–211. doi: 10.1006/viro.1998.9097. [DOI] [PubMed] [Google Scholar]
- 4.Motzko D, Ruthmann A. Eur J Cell Biol. 1984;33:205–216. [PubMed] [Google Scholar]
- 5.Rieder C L, Nowogrodzki R. J Cell Biol. 1983;97:1144–1155. doi: 10.1083/jcb.97.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stafstrom J P, Staehelin L A. Eur J Cell Biol. 1984;34:179–189. [PubMed] [Google Scholar]
- 7.Bailly E, Doree M, Nurse P, Bornens M. EMBO J. 1989;8:3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draetta, G. & Beach, D. (1989) J. Cell Sci. 12 (Suppl.), 21–27. [DOI] [PubMed]
- 9.Nurse P. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 10.Pines J, Hunter T. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- 11.Nurse P. Nature (London) 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 12.Hunt T. Nature (London) 1991;349:100–101. doi: 10.1038/349100a0. [DOI] [PubMed] [Google Scholar]
- 13.Braunagel S C, He H, Ramamurthy P, Summers M D. Virology. 1996;222:100–114. doi: 10.1006/viro.1996.0401. [DOI] [PubMed] [Google Scholar]
- 14.Belyavskyi M, Westerman M, DiMichele L, Wilson V. Virology. 1996;219:206–219. doi: 10.1006/viro.1996.0238. [DOI] [PubMed] [Google Scholar]
- 15.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 18.Hong T, Braunagel S C, Summers M D. Virology. 1994;204:210–222. doi: 10.1006/viro.1994.1525. [DOI] [PubMed] [Google Scholar]
- 19.Bozzola J J, Russell L D. Electron Microscopy. Boston: Jones & Bartlett; 1992. pp. 112–116. [Google Scholar]
- 20.Venable J H, Coggeshall R. J Cell Biol. 1965;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Lee H, Yoon D-W, Albrecht J-C, Fleckenstein B, Neipel F, Jung J U. J Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Reilly D R, Crawford A M, Miller L K. Nature (London) 1989;337:606. doi: 10.1038/337606a0. [DOI] [PubMed] [Google Scholar]
- 23.Chang Y, Moore P S, Talbot S J, Boshoff C H, Zarkowska T, Gooden-Kent D, Paterson H, Weiss R A, Mittnacht S. Nature (London) 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 24.Jung J U, Stager M, Desrosiers R C. Mol Cell Biol. 1994;14:7235–7244. doi: 10.1128/mcb.14.11.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanton C, Mann D J, Fleckenstein B, Neipel F, Peters G, Jones N. Nature (London) 1997;390:184–187. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 26.Godden-Kent D, Talbot S J, Boshoff C, Chang Y, Moore P, Weiss R A, Mittnacht S. J Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goh W C, Rogel M E, Kinsey C M, Michael S F, Fultz P N, Nowak M A, Hahn B H, Emeran M. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 28.Ooi B, Miller L K. Virology. 1988;166:515–523. doi: 10.1016/0042-6822(88)90522-3. [DOI] [PubMed] [Google Scholar]
- 29.Prikhod’ko E A, Miller L K. J Virol. 1998;72:684–692. doi: 10.1128/jvi.72.1.684-692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong Y, Zhang H, Beach D. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. Nature (London) 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 32.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 33.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J-Y, Livingston D M. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 34.Polyak K, Kato J-Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 35.Stillman B. Science. 1996;274:1659–1664. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]
- 36.Jault F M, Jault J-M, Ruchti F, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 38.Kelman Z. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- 39.Bravo R, Macdonald-Bravo H. J Cell Biol. 1987;105:1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris G F, Mathews M B. J Biol Chem. 1989;264:13856–13864. [PubMed] [Google Scholar]
- 41.Belyavskyi M, Miller J, Belyavskaya E, Wilson V G. Cytometry. 1995;21:257–264. doi: 10.1002/cyto.990210306. [DOI] [PubMed] [Google Scholar]
- 42.Cayrol C, Knibiehler M, Ducommun B. Oncogene. 1998;16:311–320. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- 43.Toschi L, Bravo R. J Cell Biol. 1988;107:1623–1628. doi: 10.1083/jcb.107.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kool M, Ahrens C H, Goldbach R W, Rohrmann G F, Vlak J M. Proc Natl Acad Sci USA. 1994;91:11212–11216. doi: 10.1073/pnas.91.23.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford A M, Miller L K. J Virol. 1988;62:2773–2781. doi: 10.1128/jvi.62.8.2773-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Possee R D, Rohrmann G F. In: The Baculoviruses. Miller L K, editor. New York: Plenum; 1997. pp. 109–140. [Google Scholar]
- 47.Fan X, McLachlin J R, Weaver R F. Virology. 1998;240:175–182. doi: 10.1006/viro.1997.8944. [DOI] [PubMed] [Google Scholar]
- 48.Kim D, Weaver R F. Virology. 1993;195:587–595. doi: 10.1006/viro.1993.1410. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Miller L K. Virology. 1995;206:314–323. doi: 10.1016/s0042-6822(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 50.Reilly L M, Guarino L A. J Gen Virol. 1994;75:2999–3006. doi: 10.1099/0022-1317-75-11-2999. [DOI] [PubMed] [Google Scholar]
- 51.Sheng Z, Charbonneau H. J Biol Chem. 1993;268:4728–4733. [PubMed] [Google Scholar]
- 52.Miller L K, Adang M J, Browne D. J Virol. 1983;46:275–278. doi: 10.1128/jvi.46.1.275-278.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]