Abstract
Integrin-linked kinase (ILK) is an ankyrin-repeat containing serine–threonine protein kinase capable of interacting with the cytoplasmic domains of integrin β1, β2, and β3 subunits. Overexpression of ILK in epithelial cells disrupts cell–extracellular matrix as well as cell–cell interactions, suppresses suspension-induced apoptosis (also called Anoikis), and stimulates anchorage-independent cell cycle progression. In addition, ILK induces nuclear translocation of β-catenin, where the latter associates with a T cell factor/lymphocyte enhancer-binding factor 1 (TCF/LEF-1) to form an activated transcription factor. We now demonstrate that ILK activity is rapidly, but transiently, stimulated upon attachment of cells to fibronectin, as well as by insulin, in a phosphoinositide-3-OH kinase [Pi(3)K]-dependent manner. Furthermore, phosphatidylinositol(3,4,5)trisphosphate specifically stimulates the activity of ILK in vitro, and in addition, membrane targetted constitutively active Pi(3)K activates ILK in vivo. We also demonstrate here that ILK is an upstream effector of the Pi(3)K-dependent regulation of both protein kinase B (PKB/AKT) and glycogen synthase kinase 3 (GSK-3). Specifically, ILK can directly phosphorylate GSK-3 in vitro and when stably, or transiently, overexpressed in cells can inhibit GSK-3 activity, whereas the overexpression of kinase-deficient ILK enhances GSK-3 activity. In addition, kinase-active ILK can phosphorylate PKB/AKT on serine-473, whereas kinase-deficient ILK severely inhibits endogenous phosphorylation of PKB/AKT on serine-473, demonstrating that ILK is involved in agonist stimulated, Pi(3)K-dependent, PKB/AKT activation. ILK is thus a receptor-proximal effector for the Pi(3)K-dependent, extracellular matrix and growth factor mediated, activation of PKB/AKT, and inhibition of GSK-3.
Phosphatidylinositol 3-kinase α(Pi(3)K) is a receptor proximal intracellular effector activated in response to a wide variety of extracellular stimuli, which include growth factors and cytokines, as well as adhesion to extracellular matrices (1, 2). The phosphatidylinositol lipid product of Pi(3)K, phosphatidylinositol(3,4,5)trisphosphate [PtdIns(3,4,5)P3] is a second messenger that acts on pathways that control cell proliferation, cell survival, protein translation, and metabolic changes, largely through the activation of protein kinase B (PKB) (also known as AKT) and p70S6 kinase (p70S6K), or the inhibition of glycogen synthase kinase 3 (GSK-3) (1, 3). PKB/AKT promotes cell survival by inhibiting apoptosis, is a proto-oncogene (4, 5), and has been shown to be activated in a Pi(3)K-dependent manner (2, 6, 7). GSK-3, on the other hand, is negatively regulated in a Pi(3)K-dependent manner upon insulin stimulation (8) and is also a critical component of the Wnt-signaling pathway (9). GSK-3 can phosphorylate highly conserved serine residues in the protein β-catenin in the presence of adematous polyposis coli and axin (9). GSK-3 also can regulate protein translation by phosphorylating and inactivating eIF2B, the guanine nucleotide exchange factor for the translation initiation factor eIF2 (3).
Pi(3)K-dependent signals therefore regulate critical cellular functions such as cell survival, cell fate determination, and protein translation via the key molecular components PKB/AKT, GSK-3, and p70S6K. Recent work (1, 11, 12) has led to a better understanding of how these components are regulated by Pi(3)K. The activation of PKB/AKT by Pi(3)K requires both the phosphoinositide products of Pi(3)K as well as protein phosphorylation of threonine-308 and serine-473 of PKB/AKT. It is now generally recognized that PtdIns(3,4,5)P3 can bind to the pleckstrin homology (PH) domain of PKT/AKT, resulting in its targeting to the plasma membrane and exposure of threonine-308. A recently identified, constitutively active kinase, PDK-1 (13, 14), then phosphorylates PKB on threonine-308. However, this phosphorylation alone is not sufficient to fully activate PKB/AKT, which also needs to be phosphorylated on serine-473 in a PtdIns(3,4,5)P3-dependent manner. PDK-1 also can directly phosphorylate p70S6K (14). Although GSK-3 can be directly phosphorylated and inactivated by PKB/AKT, GSK-3 activity also can be clearly regulated independently of PKB/AKT, for instance via activation of the Wnt pathway (9), which does not require PKB/AKT (15). We have recently identified a serine–threonine protein kinase called integrin-linked kinase (ILK-1), which can interact with the cytoplasmic domain of the β1, β2, and β3 integrin subunits (ref. 16 and G. Downie, unpublished data). Overexpression of ILK in epithelial cells results in anchorage-independent cell survival and cell cycle progression (17), as well as tumorigenesis in nude mice (18). Furthermore, ILK can induce the translocation of β-catenin to the nucleus, resulting in the formation of LEF-1 transcription factor/β-catenin complex and the enhancement of LEF-1 transcriptional activity (19). This latter finding implicates ILK in the activation of components of the Wnt-signaling pathway.
In this paper, we now report that the activity of ILK can be rapidly, but transiently, stimulated by both cell–fibronectin interactions, as well as by insulin, in a Pi(3)K-dependent manner, likely via the binding of PtdIns(3,4,5)P3 with a PH-like domain of ILK. In addition, we demonstrate that ILK not only directly phosphorylates GSK-3 and negatively regulates its activity but also can directly phosphorylate PKB/AKT on serine-473 in vitro, whereas a kinase-deficient form of ILK severely inhibits PKB/AKT serine-473 phosphorylation in vivo. These data suggest that ILK is a receptor proximal effector of Pi(3)K signals regulating the kinase activities of PKB/AKT and GSK-3.
MATERIALS AND METHODS
Stably Transfected Cells Lines.
Clones of IEC-18 cells [American Type Culture Collection (ATTC)] expressing either wild-type ILK cDNA in the sense orientation (ILK-13), or anti-sense orientation (ILK-14), or a kinase-deficient ILK cDNA (GH31R), were established as described by us (16, 19). All clones and the parental cell line were maintained in α-ME medium containing 5% fetal calf serum, insulin (10 mg/liter), and glucose (3.6 g/liter). The transfected clones also were maintained in 40 μg/ml G418 to provide selection pressure. NIH 3T3 cells (ATCC) were transfected, by using electroporation, with cDNAs-encoding haemagglutanin (HA)-tagged P110 subunits of Pi(3)K in pcDNA3 expression vectors kindly provided by M. Thelen (University of Bern, Switzerland). After selection in G418 (0.8 mg/ml), the transfected cells were cloned by limited dilution, and clones expressing HA-tagged p110 Pi(3)K were expanded and maintained in DMEM containing 10% donor calf serum and G418 (40 μg/ml).
Transient Transfections.
All transient transfection assays were carried out in 293 human embryonic kidney cells (obtained from M. Moran, University of Toronto). The cells were maintained in DMEM containing 10% donor calf serum. The cells were transfected with ILK cDNAs, HA-tagged GSK-3 cDNAs, or HA-tagged PKB/AKT cDNAs by using calcium phosphate as described by us (16). The ILK cDNAs were in p-CMV, the GSK-3 cDNAs in pcDNA3, and the PKB/AKT cDNAs were in pCMV6 plasmids. Transfections were carried out overnight, and the cells were harvested and assayed for various kinase activities 48–60 hr later.
Kinase Assays.
For kinase assays, cells were lysed in 50 mm of Hepes buffer, pH 7.5 containing NaCl (150 mM), Nonidet P-40 (1%), sodium deoxycholate (0.5%), leupeptin (10 μg/ml), phenylmethylsulfonyl fluoride (1 mM), aprotinin (2.5 μl/ml), sodium fluoride (NaF) (5 mM), and sodium orthovanadate (1 mM). Equivalent protein concentrations of cell lysates (determined by Bradford assay) were precleared with nonspecific IgG and protein A Sepharose. After centrifugation, the supernatants were immunoprecipitated with the appropriate primary antibodies as described by us (16). Kinase assays were carried out as described (16). Myelin basic protein (MBP) was used as a substrate for ILK, and glycogen synthase-1 (GS-1) peptide was used for GSK-3. Phosphorylated proteins were electrophoresed on 12% SDS/PAGE gels for detecting phosphorylated MBP and on a Tricine gel (20) for detecting phosphorylated GS-1 peptide. The gels were visualized by autoradiography or phosphorimage analysis.
Western Blot Analysis.
Equivalent protein concentrations were resolved by SDS/PAGE electrophoresis, and the proteins were electrotransfered onto poly(vinylidene difluoride) membranes. The membranes were then probed with rabbit anti-ILK antibody (16) (0.5 μg/ml), rabbit anti-GSK-3 antibody (1:400), anti-HA mAb (1 μg/ml) (Babco, Richmond, CA), or anti P-473S AKT antibody (1:1000) (New England Biolabs), and detection was by enhanced chemiluminescence.
ILK Activation.
Extracellular matrix stimulation of ILK activity was assessed in IEC-18 cells by washing the cells and incubating them for various time periods (5–60 min) in serum-free media on fibronectin (20 μg/ml) or BSA-coated plates. The cells were then lysed, and ILK activity was determined. For assessing the effect of insulin on ILK activity, IEC-18 cells were serum starved for 18 hr and then incubated with insulin (100 nM) for the indicated time periods (0–30 min). To assess the role of Pi(3)K on ILK activity, the cells were preincubated for 20 min with Pi(3)K inhibitors, Wortmannin (200 nM) (Sigma), or Ly294002 (50 μM) (Calbiochem) before plating cells on fibronectin or the addition of insulin. Quantification of the extent of phosphorylation was determined by phosphorimage analysis.
Effect of Phosphoinositides on ILK Activity in Vitro.
PtdIns(3)P, PtdIns(3,4)P2, or PtdIns(3,4,5)P3 (Matreya, Pleasant Gap, PA) were dried under nitrogen and resuspended at a concentration of 0.1 mM in Hepes (10 mM), pH 7.0 in the presence of l-α-phosphatidyl-l-serine and l-α-phosphatidylcholine, β-arachidonoyl-γ-stearoyl (1 mM each). The lipid suspensions were then vortexed and sonicated for 20 min to generate unilamellar vesicles. ILK-5-glutathione S-transferase (GST) (16) was combined with the phosphoinositide lipids (10 μM) and kinase reaction buffer containing MBP (5 μg) and [γ-32P]ATP (5 μCi). The reaction was allowed to proceed for 30 min or 2 hr at 30°C and was stopped by adding 2X sample buffer. Reaction products were analyzed by SDS/PAGE followed by autoradiography.
RESULTS
ILK Activity Is Stimulated by Fibronectin and Insulin in a Pi(3)K-Dependent Manner.
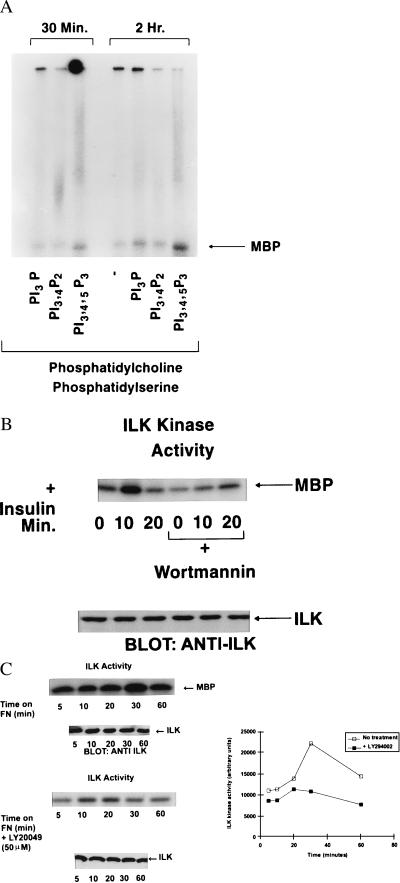
A closer examination of the amino acid sequence of ILK has revealed the presence of a sequence motif found in PH domains (21, 22) (Fig. 1). This motif has been shown to be involved in the binding of phosphatidylinositol phosphates (23, 24). Amino acids critical to the binding of such lipids to the PH domain (21) are conserved completely in ILK (Fig. 1). There is extensive sequence identity within this motif between ILK and other PH domain-containing proteins such as cytohesin-1 (a β2 integrin cytoplasmic domain-interacting protein) (24, 25) and GRP-1 (21). To determine whether 3-phosphoinositides could stimulate the protein kinase activity of ILK, the purified kinase was incubated with phospholipids, and its activity toward MBP was measured. As shown in Fig. 2A, ILK activity is stimulated in vitro by PtdIns(3, 4, 5) trisphosphate (P3) but not by PtdIns(3, 4) bisphosphate (P2), or PtdIns(3) monophosphate (P), although in some experiments ILK activity also was stimulated to a smaller extent by PtdIns(3)P. Other phosphoinositides such as PtdIns(1,4)P2 also were found to be inactive (data not shown). Because the second messenger, PtdIns(3,4,5,)P3 is specifically generated upon receptor-mediated stimulation of Pi(3)K activity, we wanted to determine whether ILK activity is stimulated in a Pi(3)K-dependent manner.
Figure 1.
Identification of phosphoinositide lipid-binding motif in ILK. Amino acid sequence alignment of ILK residues 180–212 with amino acid sequences found in the PH domains of indicated proteins. Amino acids highlighted indicate residues found to be critical for the binding of phosphatidylinositol lipids (30).
Figure 2.
(A) PtdIns(3, 4, 5)P3 specifically stimulates the activity of recombinant wild-type ILK in vitro. The indicated phospholipids were prepared as lipid vesicles as described in Materials and Methods and incubated with ILK. ILK activity was then determined by using γ32P-ATP and MBP as an exogenous substrate. Phosphorylated MBP was detected by SDS/PAGE and autoradiography. (B) Stimulation of ILK activity by insulin. IEC-18 cells were serum-starved overnight and then exposed to insulin (100 nM) for the indicated time periods. ILK was immunoprecipitated from cell extracts and ILK activity determined by using MBP as described in Materials and Methods. Pi(3)K activity was inhibited by preincubating the cells with Wortmannin (200 nM) for 20 min. Bottom shows equivalent amount of ILK in each extract as determined by Western blotting with an anti-ILK antibody. (C) Stimulation of ILK activity by cell adhesion on fibronectin. IEC-18 cells were serum-starved overnight, resuspended in serum-free medium, and allowed to adhere to fibronectin- (10 μg/ml) coated nontissue culture plastic plates for the indicated time periods. Nonadherent cells were removed, and adherent cells were lysed. ILK kinase activity was determined as described for B. Pi(3)K activity was inhibited by preincubating the cells with Ly294002 (50 μM) for 20 min. Bottom shows equivalent levels of ILK in each extract as determined by Western blotting with anti-ILK antibody. Quantification of the extent of phosphorylation was determined by phosphorimage analysis. Data shown in A, B, and C are representative of three independent experiments.
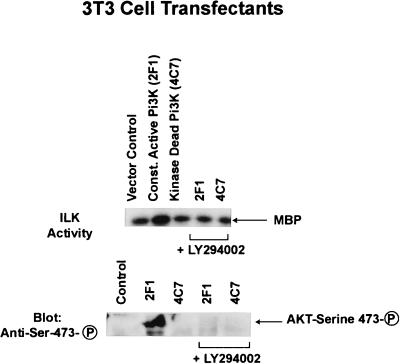
We treated quiescent, serum starved, IEC-18 intestinal epithelial cells with insulin, which is known to activate Pi(3)K. As can be seen in Fig. 2B, ILK activity is rapidly stimulated by insulin, and this activation is inhibited by prior treatment of the cells with Wortmannin (200 nM), a specific inhibitor of Pi(3)K. Another Pi(3)K inhibitor, Ly294002, also inhibits this activation (data not shown). We also show here that ILK activity is rapidly stimulated (peak activity: 20–40 min after plating) (Fig. 2C) upon attachment of cell to fibronectin; although, as shown by us (16), this activity is suppressed below that in suspension cells after 60 min on fibronectin. This transient activation of ILK is also Pi(3)K-dependent because it is inhibited by LY294002 (Fig. 2C). These data demonstrate that ILK activity is stimulated by growth factors, such as insulin, and also by cell–extracellular matrix interactions, in a Pi(3)K-dependent manner, most probably resulting from the direct interaction of Pi(3)K-generated PtdIns(3,4,5)P3 with ILK. To further demonstrate the role of Pi(3)K in ILK activation, we stably transfected NIH 3T3 cells with either constitutively activated P110 subunit of Pi(3)K (26), or a kinase-deficient mutant of Pi(3)K (26), and determined ILK activity in serum-starved, transfected clones. As shown in Fig. 3, ILK activity is higher in cells expressing constitutively active P110 subunit of Pi(3)K, than in control cells, or those expressing kinase-deficient Pi(3)K. Furthermore, the stimulated ILK activity in these cells is inhibited by prior incubation with Ly294002 (Fig. 3).
Figure 3.
Stable overexpression of HA-tagged constitutively active P110 Pi(3)K-CAAX in NIH 3T3 cells results in increased ILK activity and constitutive phosphorylation of PKB/AKT on serine-473. NIH 3T3 cell clones overexpressing constitutively active membrane-targeted p110 subunit of Pi(3)K, or kinase-deficient P110 (15), were serum-starved for 18 hr and then analyzed for ILK activity by using MBP, or for PKB/AKT serine-473 phosphorylation. Pi(3)K activity was inhibited by preincubating the cells with Ly294002 (50 μM) for 15 min. P110 expression was detected in the transfectants by Western blotting with anti-HA antibody (data not shown).
ILK Inhibits the Kinase Activity of GSK-3 and Can Phosphorylate GSK-3 in Vitro.
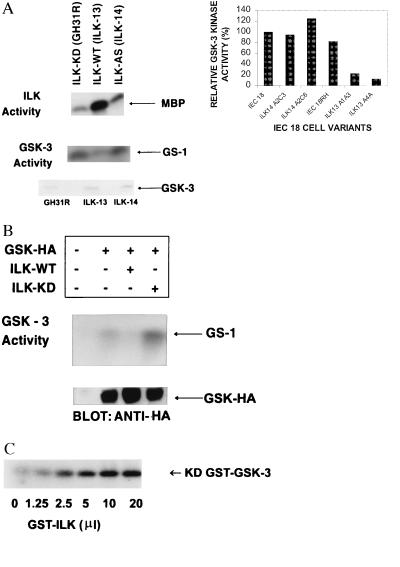
We next explored the identification of the downstream effectors of ILK. Because ILK overexpression in epithelial cells results in the translocation of β-catenin to the nucleus (19), we wanted to determine whether ILK regulates the activity of GSK-3, a kinase that normally phosphorylates β-catenin in the presence of adenomatous polyposis coli and axin. GSK-3 activity is inhibited when cells encounter Wnt, a matrix-associated protein involved in cell fate determination (10). GSK-3 activity also is inhibited by insulin. The inactivation of GSK-3 by Wnt results in the inhibition of phosphorylation of β-catenin and its subsequent stabilization and nuclear accumulation (9). It therefore seemed possible that ILK also may contribute to the nuclear localization of β-catenin by inhibiting GSK-3 activity. As shown in Fig. 4A, although GSK-3 is expressed in all IEC-18 cell transfectants, its activity is inhibited only in the ILK overexpressing ILK-13 clones (16, 19), but not in IEC-18 cells stably expressing a kinase-deficient ILK (4), or antisense ILK cDNA (16). Fig. 4A also illustrates that, as expected, ILK activity is ≈5-fold higher in ILK-13 cells compared with the control cells. GSK-3 activity also was found to be inhibited in ILK overexpressing SCP2 mammary epithelial cells (19) (data not shown). To determine whether this inhibition of GSK activity is due to ILK, we carried out transient transfection assays in human embryonal kidney epithelial cells (HEK 293). As shown in Fig. 4B, cotransfection of HA-tagged GSK-3 together with wild-type ILK resulted in inhibition of GSK-3 activity, demonstrating that kinase active ILK can inhibit GSK-3 activity. Cotransfection with kinase-deficient ILK did not result in GSK-3 inhibition but reproducibly resulted in increased GSK-3 activity over basal levels. These results suggest that the kinase-deficient ILK may be acting in a dominant-negative manner by suppressing the function of ILK. Furthermore, we also demonstrate that ILK can directly phosphorylate GSK-3 in vitro as shown in Fig. 4C, whereby coincubation of recombinant wild-type ILK with kinase-deficient recombinant GSK results in a dose-dependent phosphorylation of GSK-3. Kinase-deficient ILK did not phosphorylate GSK-3, and ILK did not phosphorylate GST-β-catenin, or GST alone (data not shown). Because GSK-3 activity can be inhibited by phosphorylation (8), it is possible that the inhibition of GSK-3 activity by ILK is due to its direct phosphorylation by ILK.
Figure 4.
ILK inhibits GSK-3 activity. (A) ILK and GSK-3 activities were measured in cell clones stably overexpressing wild-type ILK (ILK-13), kinase-deficient (KD) ILK (ILK-KD), or antisense ILK (ILK-14) (16–19) as described in Materials and Methods. Overexpression of wild-type ILK results in high ILK activity and drastic inhibition of GSK-3 activity. The level of expression of GSK-3 protein is similar in cell clones, as determined by Western blot analysis by using anti-GSK-3 antibody. (Inset) Quantification of GSK-3 activity in different IEC-18 clones. Data shown are the means of two separate experiments. (B) Transient transfection of ILK and HA-tagged GSK-3 in HEK-293 cells. Coexpression of wild-type ILK results in inhibition of GSK-3 activity whereas coexpression of kinase-deficient ILK (ILK-KD) results in enhanced GSK-3 activity relative to cells not transfected with ILK. Transfection efficiency was determined by monitoring GSK–HA expression by Western blot analysis by using anti-HA antibody. (C) ILK can phosphorylate GSK-3 in vitro. Recombinant kinase-deficient GST–GSK-3 coupled to glutathione-Sepharose beads was incubated with increasing concentrations of GST-ILK (7.5 ng/μl) in the presence of 32P-orthophosphate. After 2-hr incubation at 30°C, phosphorylated GSK was detected by SDS/PAGE and autoradiography. ILK did not phosphorylate GST.
Kinase-Deficient ILK Inhibits PKB/AKT Serine-473 Phosphorylation, and Purified ILK Promotes Serine-473 Phosphorylation of PKB/AKT in Vitro.
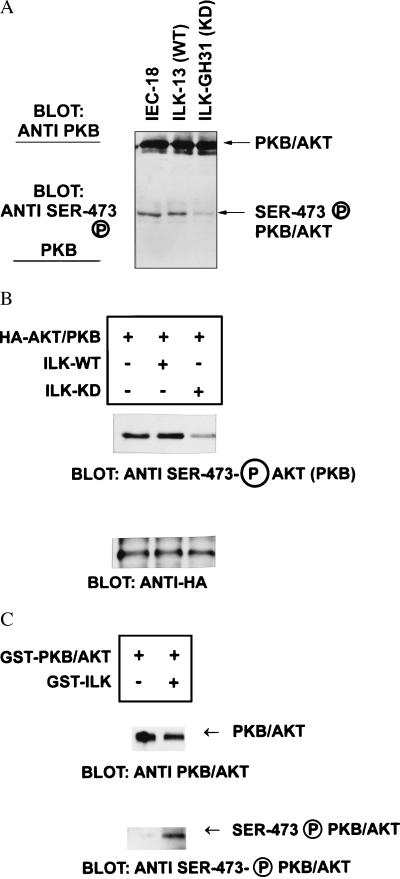
Because GSK-3 activity also can be regulated by PKB/AKT in a Pi(3)K-dependent manner (8), and because it has previously been shown by others that integrin engagement stimulates Pi(3)K activity leading to the activation of PKB/AKT (27, 2), we wanted to determine whether ILK might be upstream of PKB and may regulate its phosphorylation and activation. As phosphorylation of PKB/AKT on serine-473 is required for its activation, we monitored PKB/AKT activation by analyzing PKB/AKT serine-473 phosphorylation by using an antibody that specifically reacts with phosphorylated serine-473. The finding that in cells expressing the p110 constitutively active Pi(3)K, PKB/AKT is constitutively phosphorylated on serine-473 in the absence of serum (Fig. 3) and that in these same cells ILK activity also is constitutively stimulated suggests that ILK may regulate the serine-473 phosphorylation of PKB/AKT. As shown in Fig. 5A, PKB/AKT serine-473 phosphorylation is inhibited in IEC-18 cells stably transfected with the kinase-deficient ILK (19). Cotransfection in 293 cells of HA-tagged PKB with wild-type ILK resulted in an enhancement of phosphorylation of PKB on serine-473 (Fig. 5B), whereas cotransfection with kinase-deficient ILK resulted in a distinct inhibition of serine-473 phosphorylation (Fig. 5B). The results from both stably transfected IEC-18 cells and transiently transfected 293 cells demonstrate that the kinase-deficient ILK competes with endogenous ILK, and thus behaving in a dominant-negative manner in the regulation of phosphorylation and activation of PKB. To determine whether ILK can directly phosphorylate PKB/AKT on serine-473, we coincubated recombinant PKB/AKT with recombinant kinase active ILK in the presence of ATP and kinase reaction buffer. Phosphorylated PKB was then detected by Western blotting of the reaction products with the phosphorylated serine-473-specific antibody. As can be seen in Fig. 5C, phosphorylated GST-PKB is detected only in the presence of kinase-active ILK, demonstrating that ILK can recognize and phosphorylate serine-473 in PKB/AKT. Together with the finding that overexpression of kinase-deficient ILK can inhibit the phosphorylation of PKB/AKT on serine-473, our data suggest an important role for ILK in the activation of PKB/AKT, probably via direct phosphorylation of serine-473.
Figure 5.
Kinase-deficient ILK inhibits phosphorylation of PKB/AKT on serine-473, and wild-type ILK directly phosphorylates PKB/AKT on serine-473 in vitro. (A) The status of phosphorylation of PKB/AKT on serine-473 was determined in IEC-18 cells and in wild-type ILK overexpressing (ILK-13) cells or IEC-18 cells expressing kinase-deficient ILK (ILK-KD, GH31R) (18, 19). Cells growing in serum (5 μg/ml) containing tissue culture medium were lysed and extracts were analyzed by Western blotting by using either anti-PKB/AKT antibody or serine-473 phospho-specific antibody. PKB/AKT is expressed at equivalent levels in all cell lines but the extent of serine-473 phosphorylated PKB/AKT is dramatically inhibited in cells expressing kinase-deficient ILK. (B) Transient transfection of kinase-deficient ILK with HA-tagged PKB/AKT inhibits serine-473 phosphorylation of PKB/AKT. Cotransfection of PKB/AKT with wild-type ILK results in enhanced phosphorylation on serine-473, whereas cotransfection with kinase-deficient ILK results in inhibition of serine-473 phosphorylation of PKB/AKT. Transfection efficiency was monitored by analyzing PKB/AKT-HA expression by using Western blot analysis with anti-HA antibody. (C) Direct phosphorylation of PKB/AKT on serine-473 by ILK in vitro. Recombinant GST-PKB/AKT coupled to glutathione-Sepharose beads was incubated with or without recombinant GST-ILK (75 ng) in kinase reaction buffer. After 2 hr at 30°C, the GST-PKB/AKT beads were precipitated and analyzed by Western blot analysis. The amount of PKB/AKT detected is equivalent in the presence or absence of ILK, whereas serine-473 phosphorylated PKB/AKT is detectable only in the presence of ILK.
DISCUSSION
Cell–extracellular matrix interactions via integrins result in the stimulation of various intracellular signaling pathways leading to cell survival, cell proliferation, cell migration, and cell differentiation. While the mitogenesis signal involves the activation of the components of the ras–raf-mitogen-activated protein kinase-signaling pathway (28, 29), the pathways involved in integrin-mediated regulation of cell survival (or apoptosis suppression) and cell differentiation are not well characterized but have implicated Pi(3)K and PKB/AKT (27, 2). Recent evidence suggests an important role for the ILK (16) in regulating these pathways, as overexpression of ILK in epithelial cells induces anchorage-independent cell survival as well as an epithelial to mesenchymal transformation (19). The molecular basis for the regulation of these phenotypes by ILK is unclear, but the data presented here now suggest a mechanism for the regulation of ILK activity and the identification of its downstream effectors.
We have shown here that ILK activity can be stimulated rapidly and transiently by cell attachment to fibronectin, as well as by insulin, and that the stimulation of its activity by both these agonists is dependent on the lipid kinase, Pi(3)K. Three lines of evidence support these findings. First, we have identified within the primary amino acid sequence of ILK, sequence motifs present in PH domains known to be involved in binding phosphoinositide lipids. Amino acids shown to be critical for such binding are entirely conserved in ILK and are formed within consensus motifs identified in other functional PH domains (30). Furthermore, we have demonstrated that the second messenger product of Pi(3)K, PtdIns(3,4,5)P3, can specifically stimulate the kinase activity of ILK by several-fold in vitro. Secondly, the kinase activity of ILK is stimulated rapidly in a transient manner when epithelial cells are allowed to attach to fibronectin, or when they are exposed to insulin. Interestingly, the stimulation of ILK activity by both these agonists is inhibited by preincubation of the cells with inhibitors of Pi(3)K, namely Wortmannin, or Ly294002, providing strong evidence that the stimulation of ILK activity via integrins and receptor tyrosine kinases requires Pi(3)K activity. Lastly, ectopic expression of a membrane-bound, constitutively active form of the p110 subunit of Pi(3)K in NIH 3T3 cells results in constitutively elevated ILK activity in serum and growth factor-free conditions. The high activity is nevertheless sensitive to LY294002, clearly implicating Pi(3)K upstream of ILK.
In trying to identify downstream effectors of ILK, we focused on two serine–threonine kinases, GSK-3 and PKB/AKT, both of which have been shown to be regulated by Pi(3)K. Furthermore, we have demonstrated recently that ILK can activate the downstream components of the Wnt-signaling pathway, namely LEF-1 and β-catenin (19). Because GSK-3 has been implicated in regulating β-catenin phosphorylation and stability, it seemed to be a likely target of ILK. We have now demonstrated here that stable overexpression of kinase-active ILK in IEC-18, as well as SCP2 cells, inhibited the activity of GSK-3. Furthermore, transient transfection of GSK-3 with kinase active ILK in HEK293 cells resulted in inhibition of GSK-3 activity, whereas cotransfection of kinase-deficient ILK enhanced GSK-3 activity over basal levels. We also have shown that ILK can directly phosphorylate GSK-3 in vitro. Despite this ability of ILK to phosphorylate GSK-3 in vitro, it is not clear whether the inhibition of GSK-3 activity by ILK is due to direct phosphorylation or via the regulation of PKB/AKT activity. Because PKB/AKT phosphorylates serine-9 of GSK-3 resulting in its inhibition (8), we have attempted to determine whether overexpression of ILK in IEC-18 cells or HEK-293 cells results in GSK-3 serine-9, phosphorylation by using an anti-phospho-ser-9-GSK3 antibody. However, we have been unable to detect serine-9 phosphorylation by using this antibody. We are continuing to analyze the details of GSK-3 phosphorylation by ILK, including the stoichiometry of phosphorylation.
As discussed earlier, PKB/AKT is regulated in a Pi(3)K-dependent manner by integrin engagement (2, 27), as well as by insulin (8). The activation of PKB/AKT requires its PH domain but also is dependent on phosphorylation at two sites, threonine-308 and serine-473 (1). A constitutively active kinase, PDK-1 (13), has been shown to phosphorylate threonine-308, but the kinase responsible for phosphorylating serine-473 has not been identified. The data presented here implicate ILK as a kinase that can regulate PKB/AKT activity by phosphorylation of serine-473. We have shown that the overexpression of a kinase-deficient ILK in both stable as well as transient-transfection assays, results in a substantial inhibition of the phosphorylation of PKB/AKT on serine-473, suggesting that the kinase-deficient ILK is able to inhibit endogenous ILK function, and implicating ILK in the regulation of phosphorylation of PKB/AKT on serine-473. ILK is likely to regulate this phosphorylation directly because we can demonstrate that purified ILK can phosphorylate purified PKB/AKT on serine-473, in vitro, although the stoichiometry of phosphorylation still needs to be determined. It will be interesting to determine whether ILK also can regulate the activity of p70S6K via phosphorylation of threonine-412, which is analogous to serine-473 of PKB/AKT.
In summary, we have demonstrated a role for ILK in the regulation of two critical Pi(3)K-dependent intracellular effectors, GSK-3 and PKB/AKT. Furthermore, our findings that cell interactions with the extracellular matrix as well as insulin stimulate ILK activity in a Pi(3)K-dependent manner suggest that ILK is a receptor-proximal effector in the crossmodulation between growth factor response and integrin signaling, possibly resulting from interactions of integrins and growth factor receptors (31, 32) and their immediate effectors, such as IRS-1 (33). The results also suggest that ILK may be an important mediator of insulin-dependent responses. In this context, it is interesting to note that ILK is expressed at quite high levels in skeletal and cardiac muscle, and in the pancreas (16). The role of the direct interaction of ILK with the β1 and β3 cytoplasmic domains in the regulation of these effectors will be the immediate focus of future studies. However, our data clearly implicate ILK as a key element in the regulation of integrin, growth factor, and Wnt-signaling pathways.
Acknowledgments
We thank Marcus Thelen for Pi(3)K expression plasmids, Phillip Tsichlis for PKB/AKT expression plasmids, and Brenda Prieur for assistance in preparing the manuscript. This work was supported by grants from the National Cancer Institute of Canada (to S.D.) and from NCIC and Medical Research Council (to J.W.). S.D. is a Terry Fox Cancer Scientist of the NCIC.
ABBREVIATIONS
- ILK
integrin-linked kinase
- GSK-3
glycogen synthase kinase 3
- PKB
protein kinase B
- PH
pleckstrin homology
- HA
haemagglutanin
- GST
glutathione S-transferase
- MBP
myelin basic protein
- Pi(3)K
phosphoinositide-3 kinase
- PtdIns(3
4,5)P, P2, or P3
- phosphatidylinositol(3
4,5)monphosphate, bisphosphate, or trisphosphate
- LEF-1
lymphocyte enhancer-binding factor 1
- HEK
human embryonal kidney
References
- 1.Downward J. Science. 1997;279:673–674. doi: 10.1126/science.279.5351.673. [DOI] [PubMed] [Google Scholar]
- 2.King W G, Mattaliono M D, Chan T O, Tsichlis P N, Brugge J S. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas G, Hall M N. Curr Opin Cell Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 4.Bellacosa A, Testa J R, Staal S P, Tsichllis P N. Science. 1992;254:244–247. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J Q, Ruggeri B, Klein W M, Sonoda G, Altomare D A, Watson D K, Testa J R. Proc Natl Acad Sci USA. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 7.Marte B M, Downward J. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 8.Cross D A E, Alessi D R, Cohen P, Andjelkovic M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 9.Miller J R, Moon R T. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 10.Papkoff J, Rubinfeld B, Schryver B, Polakis P. Mol Cell Biol. 1996;16:2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toker A, Cantley L C. Nature (London) 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 12.Vanhaesebroeck B, Leevers S J, Panayoton G, Waterfield M D. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 13.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, et al. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 14.Alessi D R, Kozlowski M T, Weng Q-P, Morrice N, Auruch J. Curr Biol. 1998;8:69–81. doi: 10.1016/s0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- 15.Staveley B, Ruel L, Jin J, Stambolic V, Mastronardi F G, Heitzler P, Woodgett J R, Manoukian A S. Curr Biol. 1998;8:599–602. doi: 10.1016/s0960-9822(98)70231-3. [DOI] [PubMed] [Google Scholar]
- 16.Hannigan G E, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino M G, Radeva G, Filmus J, Bell J C, Dedhar S. Nature (London) 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 17.Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- 18.Wu C, Keightley S Y, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald I A, Dedhar S. J Biol Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- 19.Novak A, Hsu S, Leung-Hagestein C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R, Dedhar S. Proc Natl Acad Sci USA. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaggre H, von Jagow C. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 21.Klarlund J K, Guilherme A, Holik J J, Virbasius J V, Chawla A, Czech M P. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- 22.Shaw G. BioEssays. 1996;18:35–46. doi: 10.1002/bies.950180109. [DOI] [PubMed] [Google Scholar]
- 23.Salim I K, Bottomley M J, Querfurth E, Zvelebil M J, Gout I, Scaife R, Margolis R L, Gigg R, Smith C I, Driscoll P C, et al. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 24.Lemmon M A, Ferguson K M, Schlesinger J. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 25.Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, Seed B. Cell. 1996;86:233–242. doi: 10.1016/s0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 26.Didichenko S A, Tilton B, Hemmings B A, Ballmen-Hofar K, Thelen M. Curr Biol. 1996;6:1271–1278. doi: 10.1016/s0960-9822(02)70713-6. [DOI] [PubMed] [Google Scholar]
- 27.Khwaja A, Rodriguez-Viciana P, Wennsrom S, Warne P H, Downward J. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dedhar S, Hannigan G E. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Biol. 1995;11:549–600. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 30.Rameh L E, Arvidsson A K, Carraway K L, III, Couvillon A D, Rathbun G, Crompton A, VanRenterghem B, Czech M P, Ravichandran K S, Burakoff S J, et al. J Biol Chem. 1997;272:22059–22066. doi: 10.1074/jbc.272.35.22059. [DOI] [PubMed] [Google Scholar]
- 31.Woodward A S, Garcia-Cardena G, Leong M, Madri J A, Sessa W C, Languino L R. J Cell Sci. 1998;111:469–478. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto S, Teramoto H, Gutkind J S, Yamada K M. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vouri K, Ruoslahti E. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]