Abstract
Blocking αVβ3 integrin occupancy results in attenuation of the cellular migration response to insulin-like growth factor I (IGF-I). To determine whether integrin antagonists alter other IGF-I-stimulated biologic actions, quiescent smooth muscle cells (SMCs) were exposed to echistatin and their ability to respond to IGF-I was determined. Echistatin (10−7 M) inhibited IGF-I-stimulated DNA synthesis by 80%, and the protein synthesis response also was inhibited. Therefore blocking occupancy of αVβ3 inhibited multiple target cell actions of IGF-I. To determine whether blocking αVβ3 occupancy could alter IGF-I receptor-mediated signal transduction, the ability of IGF-I to stimulate phosphorylation of insulin receptor substrate-1 (IRS-1) was analyzed. A 10-min exposure to 100 ng/ml of IGF-I resulted in a substantial increase in phosphorylated IRS-1, and echistatin (10−7 M) blocked the IGF-I-induced IRS-1 phosphorylation response. Echistatin also attenuated downstream signaling because the capacity of the p85 subunit of phosphatidylinositol-3 kinase (PI-3 kinase) to bind to IRS-1 was blocked. In contrast, exposure of SMCs to vitronectin (1.0 μg/cm2) or thrombospondin (0.25 μg/cm2), two known ligands for αVβ3, resulted in enhancement of the IGF-I-stimulated IRS-1 response. To determine whether these effects were caused by alterations in receptor kinase activity, the IGF-I receptor was immunoprecipitated and then analyzed for phosphotyrosine. Echistatin (10−7 M) significantly reduced IGF-I-stimulated tyrosine phosphorylation of the IGF-I receptor β subunit. We conclude that occupancy of the αVβ3 integrin is necessary for IGF-I to fully activate the kinase activity of the IGF-I receptor and phosphorylate IRS-1. Activation of the αVβ3 receptor results in an interaction with the IGF-I signal transduction pathway, which modulates SMCs responsiveness to IGF-I.
Vascular smooth muscle cells (SMCs) have been shown to contain insulin-like growth factor I (IGF-I) receptors and respond to IGF-I with increases in DNA and protein synthesis (1–3), as well as cell migration (4, 5). More recently, IGF-I has been shown to have an antiapoptotic effect in this cell type (6). Additionally, IGF-I has been shown to interact with other stimuli of SMCs replication, such as platelet-derived growth factor (PDGF), thrombin, and angiotensin-II, to enhance cellular responsiveness (2, 7, 8). After IGF-I receptor activation, the heterotetrameric receptor that contains intrinsic tyrosine kinase activity phosphorylates two proteins that are important for signal transduction, IRS-1 and IRS-2 (9, 10). Several lines of experimental evidence have shown that phosphorylation of IRS-1 is required for certain IGF-I-mediated biologic responses (11).
Several other variables have been analyzed to determine whether they alter IGF-I responsiveness of SMCs and whether these changes might lead to changes in vessel wall responsiveness to IGF-I. Cooperative interactions with other growth factors, such as PDGF, thrombin, and angiotensin-II have been analyzed (3–8, 12). Changes in IGF-I receptor number have been determined, but they are usually minimal and are associated with a decrease in IGF-I synthesis and secretion (7). IGF-binding proteins (IGFBPs) also have been shown to be important determinants of cellular responsiveness to IGF-I (13). SMCs have been shown to synthesize and secrete three forms of IGFBPs, including IGFBP-2, -4, and -5 (14). IGFBP-2 can act as a weak stimulator of IGF-I action in the presence of high concentrations of IGF-I (15). IGFBP-4, in contrast, is usually a negative regulator of IGF-I action (16, 17). Responses to IGFBP-5 are biphasic in that, when a low concentration of this material is associated with extracellular matrix, it can act to enhance IGF-I actions, whereas when a high concentration of intact, nonproteolytically cleaved protein is present in interstitial fluids, it acts to inhibit IGF-I binding to receptors and inhibits IGF-I actions (18, 19).
In previous studies, we have determined that integrin occupancy is necessary for SMCs to migrate optimally in response to IGF-I (20). αVβ3 occupancy appears to be very important for the cellular migration response to IGF-I because these cells will migrate in the absence of serum if vitronectin alone is added to the culture plates (21) and blocking matrix protein occupancy of the αVβ3 receptor using specific αVβ3 antagonists, such as echistatin, results in attenuation of the SMCs migration response to IGF-I (21). That there could be an interaction between the αVβ3-signaling pathway or proteins that associate with the αVβ3 within the focal adhesion complex and elements in the IGF-I receptor signal transduction pathway is suggested by several reports. Uvori and Rhouslahti (22) reported that IRS-1 binds to αVβ3 after insulin receptor activation in a rat fibroblast and a pancreatic tumor cell line. In a different test system, Miyamoto, et al. (23) showed that focal clustering of β1 integrins within the focal adhesion complex led to enhanced PDGF receptor phosphorylation in response to growth factor stimulation. More recently, Senger et al. (24) showed that angiogenesis promoted by vascular endothelial growth factor was regulated through α1β1 and α2β1 integrins. These findings suggest that there may be direct interactions between growth factor receptor-signaling pathways and signaling elements that are activated by integrin occupancy; therefore, we attempted to determine whether altering ligand occupancy of αVβ3 would alter IGF-I receptor signaling.
MATERIALS AND METHODS
Materials.
Human IGF-I and des 1–3 IGF-I were purchased from Bachem. Poly(vinylidene diflouride) (PVDF) filters were purchased from Millipore. Autoradiographic film was obtained from Eastman Kodak. Fetal bovine serum, DMEM, penicillin, and streptomycin were purchased from Life Technologies, Grand Island, NY. Trypsin was obtained from Boehringer Mannheim. The anti-phosphotyrosine antibody (PY20) was obtained from Transduction Laboratories, Lexington, KY. An anti-phosphatidylinositol-3 kinase (PI-3 kinase) antibody (P-85 subunit) and an anti-IRS-1 antibody were purchased from Upstate Biotechnology, Lake Placid, NY. An anti-IRS-2 antibody was kindly provided by Morris White, Joslin Diabetes Center, Boston. Anti-αV antiserum was purchased from Chemicon. Laminin, type IV collagen, echistatin, insulin, and thrombospondin were purchased from Sigma. [3H]Thymidine was purchased from ICN. A polyclonal antibody that was specific for the IGF-I receptor β subunit was purchased from Santa Cruz Biotechnology. [35S]Methionine was purchased from New England Nuclear. Tissue culture plastic ware was purchased from Falcon Labware.
Methods.
Porcine aortic smooth muscle cells (pSMCs) were obtained from explants of thoracic aortas from young pigs by using a previously described method (25). The cells that migrated from the explants were maintained and cultured in DMEM supplemented with glucose (4.5 gm/liter), penicillin (100 units/ml), streptomycin (100 μg/ml), glutamine (4 mmol), and 10% fetal bovine serum in 10-cm tissue culture plates (Falcon 3001). The cells were used between passages 4 and 12. These cells were subcultured after trypsinization on either 24-well plates or 96-well microtiter plates in DMEM supplemented with 10% fetal bovine serum. For the protein synthesis experiments, the cells were plated at 5,000 cells/cm2. After one medium change, they were incubated for an additional 3 days and then used. For DNA synthesis experiments, the cells were plated at 5,000 cells per culture well in 96-well plates and cultured for 5 days without a medium change. To determine changes in IRS-1 phosphorylation, the cultures were seeded at 5,000 cells/cm2 on 10-cm culture dishes and then grown to confluency for ≈7 days with one medium change. In some experiments, the cells were plated on vitronectin (1.0 μg/cm2) or thrombospondin (0.25 μg/cm2), or laminin (1.0 μg/cm2) plus type IV collagen (5.0 μg/cm2) at 10,000 cells/cm2 in serum-free DMEM and allowed to attach for 4 hr before initiation of the experiment.
Immunoprecipitation of IRS-1, IRS-2, PI-3 Kinase (P85 Subunit,) and IGF-I Receptor.
To analyze the phosphorylation of IRS-1 and the IGF-I receptor, pSMCs cultures in 10-cm plates were serum deprived for 12 hr in the presence of echistatin (10−8 or 10−7 M). They were then exposed to IGF-I (100 ng/ml), insulin (10 μg/ml or 10 ng/ml), or fresh DMEM alone for 10 min at 37°C. The cells were then scraped into 1 ml of lysis buffer (1% Nonidet P-40/0.25% sodium deoxycholate/1 mM EGTA/150 mM NaCl/50 mM Tris⋅HCL, pH 7.5/1 mM sodium vanadate/1.0 mM NaF/1.0 mM PMSF/1 μg/ml pepstatin/1 μg/ml leupeptin/1.0 μg/ml aprotinin) termed RIPA buffer and centrifuged at 15,000 × g for 10 min. The proteins in the supernatant were then exposed to a 1:300 dilution of rabbit anti-IRS-1, a 1:100 dilution of rabbit anti-IRS-2, or a 1:500 dilution of rabbit anti-IGF-I receptor antibody for 14 hr at 4°C. The immune complexes were precipitated by the addition of protein A-Sepharose as previously described (18). The precipitates were analyzed by SDS/PAGE (7.5%) and then transferred to an Immobilon PSQ, 0.45-μm pore size filter (Millipore). Immunoblotting for phosphotyrosine was carried out by using a 1:1,000 dilution of (PY20) (Transduction Laboratories) anti-phosphotyrosine antibody. In some experiments, the filters were immunoblotted for IRS-1 or IGF-I receptor by using 1:500 dilutions of the two antisera that were used for immunoprecipitation. The immune complexes were detected by enhanced chemiluminescence by using the method as previously described (18).
Ligand occupancy of the α2β1 integrin was stimulated by plating the cells on culture dishes that had been coated in laminin (1.0 μg/cm2) and type IV collagen (5.0 μg/cm2). The αVβ3 integrin was stimulated by plating the cells on plates that had been coated with vitronectin by using a concentration of 1 μg/cm2 or adding 2.0 μg/ml of vitronectin to the serum free medium for 4 hr and then determining the capacity of IGF-I to stimulate IRS-1 phosphorylation as described. To determine whether the p85 subunit of PI-3 kinase would bind to IRS-1, the IRS-1 immunoprecipitates were analyzed for the presence of p85 by incubating the immunoblots of the immunoprecipitates with a 1:1,000 dilution of anti-p85 antiserum.
Measurement of [3H]Thymidine Incorporation into pSMCs.
pSMCs were plated at 5,000 cells/cm2 on 96-well culture plates. After 5 days, the cultures were exposed to increasing concentrations of IGF-I (2–50 ng/ml) in the presence or absence of echistatin (10−8 or 10−7 M) and fresh DMEM-H (0.2 ml) supplemented with 0.2% human platelet-poor plasma and 0.5 μCi of [3H]thymidine (specific activity 35 Ci/mmol). In some experiments, soluble vitronectin (2 μg/ml) also was added to the plates. After a 36-hr incubation, the plates were placed on ice and washed twice with cold phosphate-buffered saline and then incubated with cold 5% trichloroacetic acid for 10 min. The trichloroacetic acid precipitable material was solubilized in 0.1 ml of 0.1% SDS/0.1 M NaOH, and the radioactivity was quantified in the Beckman Scintillation Counter by using ScintiSafe Eco II (Fisher Scientific) as a scintillant.
Measurement of Protein Synthesis.
pSMCs were grown to confluence in 24-well culture plates (Falcon 3036). The cultures were rinsed once with serum free DMEM and incubated with 0.25 ml of low (10−7 M) methionine MEM (a mixture of 10% DMEM and 90% methionine-free DMEM) for 6 hr in the presence of 50 μCi per well of [35S]methionine (specific activity 1,206 Ci/mmol). Additional wells also received IGF-I (0–20 ng/ml) and various concentrations of echistatin between 10−9 and 10−7 M. After 6 hr the amount of [35S]methionine that had been incorporated into total protein was determined by using the extraction method described previously and scintillation counting.
Preparation of Vitronectin.
Porcine vitronectin was prepared from porcine plasma by using a previously described method (26). Purity was proven by SDS/PAGE with silver staining, which showed a single band at 67 kDa. This band was proven to be vitronectin by immunblotting using a 1:1,000 dilution of a polyclonal antibody that was purchased from Sigma.
RESULTS
DNA Synthesis Responses of SMCs to IGF-I.
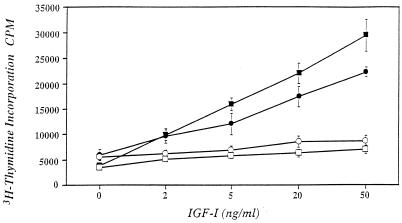
The addition of increasing concentrations of IGF-I resulted in a 3.8-fold increase in [3H]thymidine incorporation into DNA compared with control cultures (Fig. 1). If the cultures were plated on vitronectin before initiation of the experiment, the response to IGF-I was enchanced (1.5-fold increase), suggesting that ligand binding to αVβ3 modulates IGF-I stimulation of the cellular DNA synthesis response. To further confirm this conclusion, increasing concentrations of IGF-I were added with 10−7 M echistatin. As shown in Fig. 1, echistatin resulted in 80% inhibition of the ability of IGF-I (20 ng/ml) to stimulate DNA synthesis. If the cells were plated on vitronectin, echistatin inhibited their response by 86%. Importantly, this concentration of echistatin did not inhibit cell attachment, and it had no effect on basal [3H]thymidine incorporation. To further prove that these effects were specific, the responses to human serum or PDGF were assessed in the presence of echistatin. Echistatin concentrations as high as 10−7 M had no effect on the [3H]thymidine incorporation response to 10% serum (Table 1). There was a significant effect on the response to 20 ng/ml of PDGF but only a 27% reduction in this response was noted.
Figure 1.
[3H]Thymidine incorporation response to IGF-I and echistatin. Quiescent pSMCs cultures were stimulated with increasing concentrations of IGF-I (•—•) and the amount of [3H]thymidine that was incorporated into DNA determined as described in Materials and Methods. Additional cultures also were exposed to echistatin 10−7 M (□—□) plated on vitronectin (2.0 μg/well) (■—■) or plated on vitronectin and exposed to echistatin (○—○). Each point represents the mean of triplicate determinations.
Table 1.
DNA synthesis response to serum or PDGF
| [3H]Thymidine/incorporation, CPM | |
|---|---|
| Human serum 10% | 39543 ± 3886 |
| +Echistatin 10−8 M | 38774 ± 4107 |
| +Echistatin 10−7 M | 38662 ± 3943 |
| PDGF 30 ng/ml | 28986 ± 3011 |
| +Echistatin 10−8 M | 24621 ± 2462 |
| +Echistatin 10−7 M | 22499 ± 2776 |
| Platelet-poor plasma | 4539 ± 492 |
The results are the mean ± SD of triplicate determinations in three separate experiments.
Stimulation of Protein Synthesis.
To further document the effects of blocking ligand occupancy of the αVβ3 receptor on IGF-I actions, the effect of increasing concentrations of echistatin on the ability of IGF-I to stimulate protein synthesis was determined. As shown in Table 2, the addition of IGF-I between 0 and 20 ng/ml stimulated a 2.4-fold increase in [35S]methionine incorporation into protein. In contrast, if echistatin was added at 10−7 M, the response to IGF-I was inhibited by 94%, and 10−9 M resulted in a statistically significant reduction.
Table 2.
Protein synthesis responses
| Treatment | [35S]Methionine incorporation, cpm |
|---|---|
| Control | 3937 ± 504 |
| IGF-I 10 ng/ml | 5719 ± 499 |
| IGF-I 20 ng/ml | 6943 ± 821 |
| IGF-I 50 ng/ml | 8862 ± 669 |
| IGF-I 50 ng/ml + echistatin 10−9 M | 7318 ± 801 |
| IGF-I 50 ng/ml + echistatin 10−8 M | 5004 ± 662 |
| IGF-I 50 ng/ml + echistatin 10−7 M | 4001 ± 390 |
| Echistatin 10−7 M | 3788 ± 422 |
The results are the mean ± SD of triplicate determinations in two separate experiments.
Inhibition of Ligand Occupancy of αVβ3 Results in Decreased IGF-I-Stimulated IRS-1 Phosphorylation.
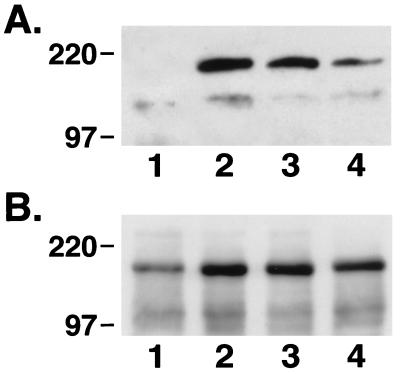
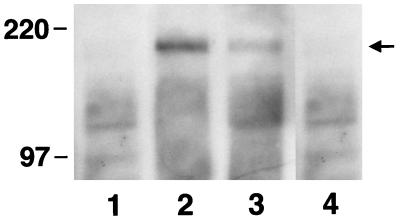
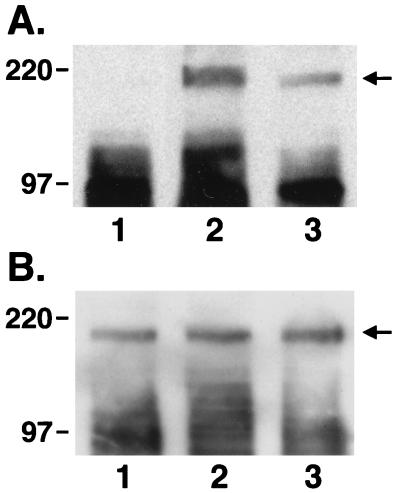
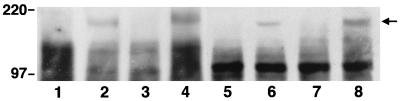
To determine whether these effects of echistatin were mediated through an alteration in IGF-I receptor-mediated signal transduction, confluent, quiescent cultures were exposed to IGF-I alone or the combination of IGF-I plus either 10−8 or 10−7 M echistatin for 10 min. Echistatin was added 12 hr before the addition of IGF-I. After a 10-min incubation at 37°C, the cells were lysed and IRS-1 was immunoprecipitated, and then the immunoprecipitated proteins were separated by SDS/PAGE and immunoblotted for phosphotyrosine. IGF-I stimulated tyrosine phosphorylation of a band with an Mr estimate of 175 kDa (Fig. 2A). The band was shown to be IRS-1 by immunoblotting (Fig. 2B). The addition of 10−8 M echistatin resulted in a 35% inhibition of signal intensity of tyrosine phosphorylated IRS-1, and 10−7 M resulted in >70% inhibition (Fig. 2A). The control immunoblot for IRS-1 abundance showed that the signal intensity was nearly identical in all lanes. To further determine whether modulation of αVβ3 occupancy would affect the ability of IGF-I to induce IRS-1 phosphorylation, SMCs were plated on vitronectin or thrombospondin coated plates for 4 hr to allow cell attachment and then stimulated with IGF-I. Exposure to vitronectin or thrombospondin resulted in major increases in the degree of tyrosine phosphorylation of IRS-1 in response to IGF-I. Compared with cultures that were plated on laminin/type IV collagen, which bind to the α2β1 integrin, the IGF-I-induced increase in IRS-1 phosphorylation was enhanced by 2.4-fold (vitronectin) and 2.2-fold (thrombospondin). Insulin, at 10 μg/ml, also stimulated the phosphorylation of IRS-1, and the insulin-induced increase in phosphorylation was inhibited by exposure to 10−7 M echistatin. Because high concentrations of insulin will interact with the IGF-I receptor, we tested the effect of 10 ng/ml of insulin. This concentration failed to stimulate the phosphorylation of IRS-1 (Fig. 4).
Figure 2.
Effects of echistatin on tyrosine phosphorylation of IRS-1 after IGF-I stimulation. Subconfluent SMCs were incubated without echistatin (lanes 1 and 2) or with echistatin at 10−8 M (lane 3) or 10−7 M (lane 4) for 12 h in serum-free DMEM-H. The cells were exposed to no IGF-I (lane 1) or 100 ng/ml IGF-I (lanes 2–4) for 10 min. They were lysed with 1 ml of RIPA buffer, and the lysates were immunoprecipitated by using an anti-IRS-1 antiserum as described in Materials and Methods. The precipitated proteins were separated by 7.5% SDS/PAGE and transferred to a PVDF membrane. The membrane was blotted with anti-phosphotyrosine antibody (A) or an antibody against IRS-1 (B).
Figure 4.
Effects of echistatin on tyrosine phosphorylation of IRS-1 after insulin stimulation. Subconfluent SMCs were incubated without echistatin (lanes 1, 2, and 4) or with echistatin at 10−7 M (lane 3) for 12 hr in serum-free DMEM-H. The cells were exposed to no insulin (lane 1) or 10 μg/ml insulin (lanes 2 and 3) or 10 ng/ml insulin (lane 4) for 10 min. After lysis with 1 ml of RIPA buffer, the lysates were immunoprecipitated by using an anti-IRS-1 antiserum as described in Materials and Methods. The precipitated proteins were separated by 7.5% SDS/PAGE and transferred to a PVDF membrane. The membrane was blotted with anti-phosphotyrosine antibody.
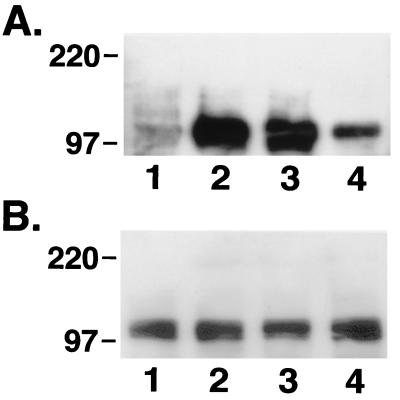
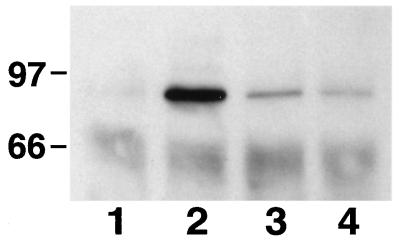
To further define the mechanism by which this interaction occurred, we exposed the cultures to IGF-I or IGF-I plus echistatin (10−8 or 10−7 M) and then immunoprecipitated the IGF-I receptor from the cell lysates. As shown in Fig. 5, IGF-I stimulated phosphorylation of the β subunit of the receptor, and this response was inhibited by 70% with 10−7 M echistatin. A control immunoblot showed that similar amounts of the IGF-I receptor were immunoprecipitated. Although 100 ng/ml IGF-I was used, in additional experiments we determined that 25 and 50 ng/ml were equipotent in stimulating tyrosine phosphorylation of IGF-I receptor. The addition of similar concentrations of des 1–3 IGF-I stimulated the tyrosine phosphorylation of the receptor to the same extent compared with native IGF-I (data not shown). To determine whether blocking IGF-I receptor phosphorylation would alter phosphorylation of IRS-2, another important substrate of the receptor tyrosine kinase, IRS-2 phosphorylation was measured following cellular exposure to IGF-I or IGF-I plus echistatin. As shown in Fig. 6, IRS-2 phosphorylation also was inhibited by echistatin. To determine whether echistatin exposure would alter downstream signaling, we determined the capacity of the p85 subunit of PI-3 kinase to bind to IRS-1 after cellular exposure to IGF-I or IGF-I plus echistatin. As shown in Fig. 7, p85 binding to IRS-1 was inhibited by echistatin exposure. To investigate the possibility that the effect of echistatin was due to blocking a direct physical association between IRS-1 or the IGF-I receptor and αVβ3, we attempted to determine whether IRS-1 or the IGF-I receptor and αVβ3 would coprecipitate after IGF-I stimulation. The cell lysates were immunoprecipitated with anti αV antiserum then immunoblotted for IRS-1 or the IGF-I receptor. Both experiments were repeated three times, but in no case, were we able to demonstrate coprecipitation by using the same conditions that were effective for altering IRS-1 and IGF-I receptor phosphorylation.
Figure 5.
Effect of echistatin on tyrosine phosphorylation of IGF-I receptor after IGF-I stimulation. Subconfluent SMCs were incubated without echistatin (lanes 1 and 2) or with echistatin at 10−8 M (lane 3) and 10−7 M (lane 4) for 12 h in serum-free DMEM-H. The cells were exposed to no IGF-I (lane 1) or 100 ng/ml IGF-I (lanes 2–4) for 10 min. The cells were lysed, and the lysate was immunoprecipitated by using an anti-IGF-I receptor β subunit antiserum. The precipitated proteins were separated by 7.5% SDS/PAGE and transferred to a PVDF membrane. The membrane was blotted with an antibody against phosphotyrosine (A) or an antibody against IGF-I receptor β subunit (B).
Figure 6.
Effect of echistatin on tyrosine phosphorylation of IRS-2 after IGF-I stimulation. Subconfluent SMCs were incubated without echistatin (lanes 1 and 2) or with echistatin at 10−7 M (lane 3) for 12 h in serum-free DMEM-H. The cells were exposed to no IGF-I (lane 1) or 100 ng/ml (lanes 2 and 3) for 10 min. They were then lysed with 1 ml of RIPA buffer, and the lysates were immunoprecipitated by using an anti-IRS-2 antiserum as described in Materials and Methods. The precipitated proteins were separated by 7.5% SDS/PAGE and transfered to a PVDF membrane. The membrane was blotted with anti-phosphotyrosine antibody (A) or an antibody against IRS-2 (B).
Figure 7.
Effect of echistatin on the association of IRS-1 and PI-3 kinase. Subconfluent SMCs were incubated without echistatin (lanes 1 and 2) or with echistatin at 10−8 M (lane 3) and 10−7 M (lane 4) for 12 h in serum-free DMEM-H. The cultures were then exposed to no IGF-I (lane 1) or 100 ng/ml IGF-I (lanes 2, 3, and 4) for 10 min. They were lysed, and the lysate was immunoprecipitated by using an anti-IRS-1 antiserum. The precipitated proteins were separated by 7.5% SDS/PAGE and transferred to a PVDF membrane. The membrane was blotted with an anti-P-85 antibody as described in Materials and Methods.
DISCUSSION
The results of this study clearly demonstrate that blocking ligand occupancy of αVβ3 results in attenuation of SMCs responsiveness to IGF-I. Although it might be predicted that blocking αVβ3 occupancy would inhibit IGF-I-stimulated cell migration simply by blocking physical contact of αVβ3 with extracellular matrix proteins, which has been postulated to be necessary for the physical forces that mediate migration, it was not intuitively obvious that blocking ligand occupancy of this receptor would inhibit other IGF-I-stimulated responses. Therefore, we tested whether blocking ligand occupancy of αVβ3 would block the IGF-I stimulation of protein and DNA synthesis. These responses were inhibited by echistatin, a known competitive antagonist of αVβ3 ligand occupancy in this cell type. Importantly, echistatin had no effect on basal [3H]thymidine or [35S]methionine incorporation. It also had no effect on the serum stimulated responses, suggesting that this inhibition was not a result of direct cytotoxicity. Similarly, cell detachment was not observed. These results suggested that there was a specific interaction between activation of the αVβ3 integrin receptor and the IGF-I receptor-mediated signal transduction pathways.
To determine whether this was the case, we analyzed the effect of blocking αVβ3 occupancy on the phosphorylation of the β subunit of the IGF-I receptor and IRS-1. IRS-1 is the most proximal element in the IGF-I receptor signal transduction pathway. It has been shown to be directly phosphorylated on tyrosines by the IGF-I receptor tyrosine kinase in response to IGF-I receptor ligand occupancy (9, 11, 27), and the degree of phosphorylation is dependent on the degree of stimulation of intrinsic tyrosine kinase activity of the receptor (27). In this study, we demonstrate that concentrations of echistatin, for example 10−7 M, that are effective in inhibiting ligand activation of αVβ3 in this cell type (20) are also effective in inhibiting optimal activation of the IGF-I receptor tyrosine kinase, IRS-1, and IRS-2. This implies that an interaction involving activation of the αVβ3 receptor and the IGF-I-signaling pathway is required for IGF-I to fully stimulate its anabolic effects. This was further confirmed by showing that plating the cells on a matrix that was enriched in vitronectin resulted in augmentation of the cellular DNA synthesis response to IGF-I above what could be achieved by maintaining the cells in 0.2% serum, and augmentation of the IRS-1 phosphorylation response to IGF-I also was observed. Because we have shown previously that 0.2% serum or vitronectin must be present for these cells to respond to IGF-I with increased migration (21), this suggests that augmentation of IRS-1 phosphorylation by ligand occupancy of αVβ3 is required for all pSMCs responses to IGF-I.
The molecular mechanism by which αVβ3 receptor occupancy augments IGF-I stimulation of IRS-1 phosphorylation was addressed in these studies. Uvori and Rhouslahti (22) reported that activation of the insulin receptor in fibroblasts that had been transfected with the insulin receptor and a pancreatic tumor cell line that had been transfected with αV led to binding of phosphorylated IRS-1 to αVβ3 and that ligand occupancy of both receptors was required for this binding event to occur. We attempted to demonstrate coprecipitation of activated αVβ3 and the IGF-I receptor and IRS-1 but were unsuccessful. Specifically, we treated cells with increasing concentrations of IGF-I and showed that IRS-1 and the receptor were phosphorylated, but we were unable to coprecipitate by using an antibody that has been shown previously to coprecipitate IRS-1 with other proteins, such as PI-3 kinase, in this test system (11, 18). This difference between our findings and those of Uvori and Rhoushalti may be because of the fact that we used nontransfected cells that had a lower IGF-I receptor number. Our results suggest that in our nontransfected SMCs there is no direct physical association between αVβ3 and the IGF-I receptor.
Studies by Miyamato and coworkers (23) showed that the degree of epidermal growth factor, PDGF, or fibroblast growth factor receptor tyrosine phosphorylation could be enhanced if β1 integrin occupancy was stimulated. Specifically, these investigators activated unidentified β1 integrins by plating cells on anti-β1 antibody-coated beads and measuring tyrosine phosphorylation of the receptors in response to growth factor stimulation. They did not report coimmunoprecipitation of the receptors and β1 integrins but postulated that there is a close physical association of the two types of receptors within the focal adhesion complex. They proposed that clustering of β1 integrins leads to enhanced ligand induced activation of these growth factor receptors, possibly due to enhanced transphosphorylation within the focal adhesion complex (23). Other structural elements of the focal adhesion complex that have been shown to localize under such conditions include SRC kinase, focal adhesion kinase, PI-3 kinase, and cytoskeletal proteins such as vincullin and tallin (28, 29). Because we were unable to detect binding of αVβ3 to the IGF-I receptor, this suggests that the mechanism proposed by Miyamoto et al. (23) by which ligand induced clustering of integrins activates growth factor receptor tyrosine kinases is the most likely explanation for our findings.
To determine whether IGFBPs are involved in the alteration of IGF-I signaling, the IGF-I receptor phosphorylation response to 25 and 50 ng/ml of IGF-I was analyzed. These lower concentrations were equally effective, and des 1–3 IGF-I, an IGF-I analog, that binds to IGF-I receptor but binds weakly to IGFBPs induced a similar response. These results show that under the conditions used in these experiments, it is unlikely that IGFBPs contribute to the altered IGF-I signaling.
We also investigated the activation of the downstream-signaling element, PI-3 kinase. Blocking αVβ3 occupancy blocked the binding of the P85 subunit of PI-3 kinase to IRS-1, suggesting that its activation can be modulated by αVβ3. Myers et al. (11) have shown p85 binding to IRS-1 after IGF-I receptor activation. Two signaling pathways are generally stimulated by IGF-I receptor activation. These include the Grb-2 mitogen-activated protein kinase activation pathway, which is necessary for growth stimulation by IGF-I and the PI-3 kinase protein tyrosine kinase B pathway, which has been shown to be necessary for activating the transcription of specific genes in response to IGF-I (30, 31). Whether the three processes that we have shown to be sensitive to αVβ3 blockade utilize one or both of these pathways has not been determined. However other investigators have determined clearly that the Grb-2-mitogen-activated protein kinase pathway is necessary for mitogenesis to proceed normally (32), and PI-3 kinase activation is necessary to stimulate membrane ruffling, an important component of the cell migration response (33). This suggests that blocking IRS-1 phosphorylation in this cell type may affect signaling through both pathways, but this remains to be definitively determined.
The molecular mechanism by which αVβ3 actually functions to alter IGF-I receptor phosphorylation could be mediated through multiple types of interactions. Because these two proteins do not appear to coimmunoprecipitate, it is possible that these elements colocalize with focal adhesion complexes. It is also possible that αVβ3 occupancy by echistatin directly affects an intermediary protein that binds to the IGF-I receptor that interacts directly with the tyrosine kinase domain to limit its ability to phosphorylate IRS-1 or that it binds directly to IRS-1 and limits its ability to function as a substrate. A candidate protein would be integrin-activating protein. Integrin-activating protein is a transmembrane protein that has been shown to be activated after αVβ3 occupancy and binds specifically to αVβ3 (34). At present, it is not known whether SMCs contain an integrin activating protein, but if they do, this would be a candidate protein for mediating the interaction between αVβ3 and IRS-1 or between αVβ3 and the tyrosine kinase subunit of the IGF-I receptor.
Although blocking αVβ3 occupancy had some effect on PDGF responsiveness of the cells, this effect was not as great as that shown with IGF-I. This result suggests that different growth factors may be sensitive to blocking ligand occupancy of different and specific integrins. Ross and coworkers (35) have shown that blocking the α2β1 occupancy reduces cell migration response to PDGF. On the other hand, because vascular SMCs are essentially devoid of insulin receptors (36), the phosphorylation of IRS-1 induced by 10 μg/ml insulin may be due to the interaction of insulin with the IGF-I receptor. This was further confirmed by showing that 10 ng/ml, a concentration of insulin that activates only the insulin receptor, failed to stimulate the phosphorylation of IRS-1.
Ligand occupancy of αVβ3 is a particularly attractive candidate for analysis because it is expressed in the SMCs within vessel walls and because blocking its occupancy appears to have some effect on the progression of atherogenesis (36). Several extracellular matrix proteins that bind avidly to αVβ3 are also present within atherosclerotic lesions. Two that appear to be increased within lesions are thrombospondin and osteopontin (38, 39). Thrombospondin is a complex protein that has multiple-binding domains, one of which interacts with αVβ3 and one of which interacts directly with integrin activating protein (34, 39). This suggests that stimulation by thrombospondin binding would result in full activation of αVβ3. Thrombospondin expression is enhanced during formation of the neointima (40). Because our data show that thrombospondin augments IGF-I stimulated phosphorylation of IRS-I in cultured SMCs, this may provide a mechanism by which this matrix protein contributes to the formation of neointima.
In summary, we have demonstrated that ligand occupancy of αVβ3 is required for full activation of the IGF-I receptor β subunit and the signal transduction element, IRS-1, by IGF-I stimulation of its receptor. In addition, we have demonstrated that this blockage leads to global inhibition of IGF-I action in this cell type. This suggests that blocking ligand occupancy of αVβ3 may inhibit cellular mitogenesis and anabolic response mechanisms that depend on growth factor-mediated pathways and that cooperativity between the integrin receptor occupancy and growth factor receptor occupancy is an important component of the cellular response to injury. Future studies should be directed toward identifying the exact components of the molecular mechanism that are required for this interaction to occur.
Figure 3.
Effect of ECM proteins on tyrosine phosphorylation of IRS-1. SMCs were cultured on laminin plus type IV collagen (lanes 1 and 2) or vitronectin (lanes 3 and 4). In a second experiment, they were plated on laminin plus type IV collagen (lanes 5 and 6) or thrombospondin (lanes 7 and 8) for 4 h as previously described. The cells were then exposed to no IGF-I (lanes 1, 3, 5, and 7) or 100 ng/ml IGF-I (lanes 2, 4, 6, and 8) for 10 min. The cells were lysed, and the lysates were immunoprecipitated by using an antiserum against IRS-1. The precipitated proteins were separated by 7.5% SDS/PAGE and transferred to a PVDF membrane. The membrane was blotted with an anti-phosphotyrosine antibody. The results expressed as scanning units are: lane 2, 10457; lane 4, 25326; lane 6, 9789; and lane 8, 21030.
Acknowledgments
We thank Ms. Catherine Rees for her technical assistance. We thank Mr. George Mosley for his help in preparing the manuscript. This work was supported by a grant from the National Institutes of Health HL-56850.
ABBREVIATIONS
- IGF-I
insulin-like growth factor-I
- IRS-1
insulin receptor substrate 1
- SMCs
smooth muscle cells, pSMCs, porcine aortic SMCs
- IGFBP
IGF-binding protein
- PDGF
platelet-derived growth factor
- PVDF
poly(vinylidene diflouride)
- PI-3 kinase
phosphatidylinositol-3 kinase
References
- 1.Pfeifle B, Boeder H, Ditschuneit H. Endocrinology. 1987;120:2251–2258. doi: 10.1210/endo-120-6-2251. [DOI] [PubMed] [Google Scholar]
- 2.Clemmons D R. J Cell Physiol. 1984;121:425–430. doi: 10.1002/jcp.1041210222. [DOI] [PubMed] [Google Scholar]
- 3.Bornfeldt K E, Arnqvist H J, Capron L. Diabetologia. 1992;35:104–108. doi: 10.1007/BF00402540. [DOI] [PubMed] [Google Scholar]
- 4.Bornfeldt K E, Raines E W, Nakano T, Graves L M, Krebs E G, Ross R. J Clin Invest. 1994;93:1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gockerman A, Jones J I, Prevette T, Clemmons D R. Endocrinology. 1995;136:4168–4173. doi: 10.1210/endo.136.10.7545099. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M R, Evan G I, Schwartz S M. J Clin Invest. 1995;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delafontaine P, Anwar A, Lou H, Ku L. J Clin Invest. 1996;97:139–145. doi: 10.1172/JCI118381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delafontaine P, Lou H. J Biol Chem. 1993;268:16866–16870. [PubMed] [Google Scholar]
- 9.Sun X J, Rothenberg P, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. Nature (London) 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 10.Araki E, Lipes M A, Patti M E, Bruning J C, Haag B, III, Johnson R S, Kahn C R. Nature (London) 1994;372:128–129. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 11.Myers M G, Jr, Sun X J, Cheatham B, Jachna B R, Glasheen E M, Bacher J M, White M F. Endocrinology. 1993;132:1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- 12.Delafontaine P, Lou H, Alexander R W. Hypertension. 1991;18:742–747. doi: 10.1161/01.hyp.18.6.742. [DOI] [PubMed] [Google Scholar]
- 13.Jones J I, Clemmons D R. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 14.Duan C, Hawes S, Prevette T, Clemmons D R. J Biol Chem. 1996;271:4280–4288. doi: 10.1074/jbc.271.8.4280. [DOI] [PubMed] [Google Scholar]
- 15.Bourner M J, Busby W H, Siegel N R, Krivi G G, McCusker R H, Clemmons D R. J Cell Biochem. 1992;48:215–226. doi: 10.1002/jcb.240480212. [DOI] [PubMed] [Google Scholar]
- 16.Chernausek S D, Smith C E, Duffin K L, Busby W H, Wright G, Clemmons D R. J Biol Chem. 1995;270:11377–11382. doi: 10.1074/jbc.270.19.11377. [DOI] [PubMed] [Google Scholar]
- 17.Conover C A, Keifer M C, Zapf J. J Clin Invest. 1993;91:1129–1137. doi: 10.1172/JCI116272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai Y, Busby W H, Smith C E, Clark J B, Horvitz G D, Rees C, Clemmons D R. J Clin Invest. 1997;100:2596–2605. doi: 10.1172/JCI119803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng B, Duan C, Clemmons D R. J Biol Chem. 1998;273:8994–9000. doi: 10.1074/jbc.273.15.8994. [DOI] [PubMed] [Google Scholar]
- 20.Jones J I, Prevette T, Gockerman A, Clemmons D R. Proc Natl Acad Sci USA. 1996;93:2482–2487. doi: 10.1073/pnas.93.6.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones J I, Doerr M E, Clemmons D R. Prog Growth Factor Res. 1995;6:19–27. doi: 10.1016/0955-2235(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 22.Vuori K, Ruoslahti E. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Teramoto H, Gutkind J S, Yamada K M. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senger D R, Claffey K P, Benes J E, Perruzzi C A, Sergiou A P, Detmar M. Proc Natl Acad Sci USA. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross R. J Cell Biol. 1971;50:172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- 27.Blakesley V A, Scrimgeour A, Esposito D, LeRoith D. Cytokine Growth Factor Rev. 1996;7:153–159. doi: 10.1016/1359-6101(96)00015-9. [DOI] [PubMed] [Google Scholar]
- 28.Plopper G E, McNamee H P, Dike L E, Bojanowski K, Ingber D E. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dedhar S, Hannigan G E. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 30.White M F, Kahn C R. J Biol Chem. 1994;69:1–4. [PubMed] [Google Scholar]
- 31.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G F, Holmes A B, Gaffney P R J, Reese C B, McCormick F, Tempst P, et al. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 32.Chen H C, Guan J L. J Biol Chem. 1994;264:31229–31233. [PubMed] [Google Scholar]
- 33.Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao A G, Lindberg F P, Dimitry J M, Brown E J, Frazier W A. J Cell Biol. 1996;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skinner M P, Raines E W, Ross R. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- 36.Arnqvist, H. J., Bornfeldt, K. E., Chen, Y. & Lindstrom, T. (1995) Metabolism 44, Suppl. 4, 58–66. [DOI] [PubMed]
- 37.Choi E T, Engel L, Callow A D, Sun S, Trachtenberg J, Santoro S, Ryan U S. J Vasc Surg. 1994;19:125–134. doi: 10.1016/s0741-5214(94)70127-x. [DOI] [PubMed] [Google Scholar]
- 38.Giachelli C M, Bae N, Almeida M, Denhardt D T, Alpers C E, Schwartz S M. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao A G, Lindberg F P, Finn M B, Blystone S D, Brown E J, Frazier W A. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 40.Liau G, Winkles J A, Cannon M S, Kuo L, Chilian W M. J Vasc Res. 1993;30:327–332. doi: 10.1159/000159014. [DOI] [PubMed] [Google Scholar]