Abstract
The HIV-1 Nef protein is important for pathogenesis, enhances viral infectivity, and regulates the sorting of at least two cellular transmembrane proteins, CD4 and major histocompatibility complex (MHC) class I. Although several lines of evidence support the hypothesis that the Nef protein interacts directly with the cellular protein sorting machinery, the sorting signal in HIV-1 Nef has not been identified. By using a competition assay that functionally discriminates between dileucine-based and tyrosine-based sorting signals, we have categorized the motif through which Nef interacts with the sorting machinery as dileucine-based. Inspection of diverse Nef proteins from HIV-1, HIV-2, and simian immunodeficiency virus revealed a well-conserved sequence in the central region of the C-terminal, solvent-exposed loop of Nef (E/DXXXLφ) that conforms to the consensus sequence of the dileucine-based sorting motifs found in cellular transmembrane proteins. This sequence in NefNL4-3, ENTSLL, functioned as an endocytosis signal when appended to the cytoplasmic tail of a heterologous protein. The leucine residues in this motif were required for the interaction of full-length Nef with the dileucine-based sorting pathway and were required for Nef-mediated down-regulation of CD4. These leucine residues were also required for optimal viral infectivity. These data indicate that a dileucine-based sorting signal in Nef is utilized to address the cellular sorting machinery. The data also suggest that an influence on the distribution of cellular transmembrane proteins may mechanistically unite two previously distinct properties of Nef: down-regulation of CD4 and enhancement of viral infectivity.
The nef gene product of primate lentiviruses is a critical determinant of disease progression and pathogenesis (1, 2). However, this protein appears to play three distinct roles: enhancement of viral infectivity and replication (3–7); down-regulation of cell surface proteins, specifically CD4 (8, 9) and major histocompatibiliy complex (MHC) class I (10); and alteration of T cell signaling pathways (11–13).
Of these properties, Nef-mediated down-regulation of CD4 is the best characterized mechanistically and is a consequence of enhanced endocytosis of CD4 from the cell surface (14). Nef increases by ≈2-fold the formation of clathrin-coated pits at the cell surface, and these pits specifically incorporate CD4 (15). Nef itself colocalizes with components of the clathrin-coated pit adaptor complexes (16, 17). This colocalization is independent of the presence of CD4, suggesting that Nef contains a domain that is either directly or indirectly responsible for interaction with the endocytic machinery. Additional evidence for this hypothesis is provided by the observation that fusion of Nef to the transmembrane and extracellular domains of either CD4 or CD8 induces endocytosis of the chimeric proteins (18, 19).
Nef may physically connect CD4 with the endocytic machinery (18, 20). NMR spectroscopy of Nef in the presence of a CD4-derived peptide has described a putative CD4 binding site on Nef (21). Further evidence for a direct interaction of Nef with CD4 has been derived from yeast two hybrid assays (22) and from coimmunoprecipitation of Nef and CD4 when coexpressed in insect cells (23). Recently, yeast two-hybrid assays have demonstrated that HIV-1 Nef associates weakly with the μ-chains of adaptor protein complexes 1 and 2 (17, 20). Analysis of truncation mutants has roughly mapped this interaction to amino acids 143–170 of NefHIV/LAI (17). Both x-ray crystallographic and NMR spectroscopic data describe an unstructured loop in this C-terminal region of the Nef protein, which appears available for interaction with a ligand (24, 25). This flexible loop is exposed to solvent and is physically separate from the putative CD4-binding surface located on the well-folded globular core of Nef (21).
If Nef interacts directly with the cellular endocytic machinery, it seems likely that this interaction would be mediated by a viral sequence homologous to one or more cellular sequences known to mediate similar interactions. In this regard, the internalization of transmembrane proteins from the plasma membrane and the sorting of transmembrane proteins directly from the trans-Golgi to lysosomal vesicles can be mediated by several types of peptide sequences (26). One type of sorting signal, a tyrosine-based motif, conforms to the consensus sequence YXXφ, where φ is an amino acid residue with a bulky, hydrophobic side chain (27). A second type of sorting signal, a dileucine-based motif, conforms to the consensus sequence E/DXXXLφ (28, 29). Although the only absolute requirement for this motif is the first of the two consecutive leucines, an acidic residue four amino acids upstream is favorable for endocytic function. These two sorting signals use distinct elements of the endocytic machinery that are independently saturable (30). To demonstrate this independence, fusion proteins containing the extracellular and transmembrane domains of the interleukin 2 (IL-2) receptor α chain (Tac antigen) and cytoplasmic domains containing known endocytosis signaling motifs were used in competition assays with proteins that contained similar motifs. Only proteins with similar motifs were able to inhibit each others endocytosis. This inhibition resulted in displacement of the indicator protein to the cell surface, where its increased expression could be detected by using flow cytometry. Similar competition assays are utilized in the present study to address the possible existence of an endocytosis signal in the Nef protein of HIV-1. Here, a dileucine-based sorting signal is identified in Nef within the C-terminal solvent-exposed loop, and its involvement in CD4 down-regulation and enhancement of viral infectivity and replication is demonstrated.
MATERIALS AND METHODS
Expression Vectors and Proviral Constructs.
The CD4 expression vector pCMX-CD4 was provided by Didier Trono (14). The vectors pCDM8-TAC, pTAC-DKQTLL, pTTMb, and pCDM8-LAMP1 were provided by Juan Bonifacino (30). The vectors pTAC-ENTSLL and pTAC-ENTSAA were prepared by an overlapping PCR strategy by using primers encoding these motifs to substitute the DNA sequence encoding the peptide DKQTLL of pTAC-DKQTLL with sequences encoding ENTSLL or ENTSAA. The nef gene was subcloned from the HIV-1 proviral clone pNL4-3 (31) into the expression vector pCIneo (Promega) by PCR; this construct was designated pCINL. Mutations were introduced into pCINL by using an overlapping PCR strategy and mutant primers. For the construction of proviral plasmids, mutant nef alleles were subcloned from the pCIneo-based expression vectors directly into pNL4-3. The sequences of all constructs were verified by nucleotide sequence analysis.
Transient Transfections.
293 cells were transfected with the DNAs of interest by using the reagents of the Pharmacia CellPhect Transfection Kit according to the manufacturer’s instructions. The amounts of total DNA in each transfection were standardized as necessary by the addition of pCIneo parental plasmid. A gfp expression vector, phGFP-S65T (Clontech) was included in each transfection as a control for efficiency of transfection.
Cell-Surface Staining and Fluorescence-Activated Cell Sorter (FACS) Analysis.
Cells were removed from plates with 1 mM EDTA/1× PBS 36–48 h after transfection, washed in 1× PBS/1% azide/2% fetal calf serum, stained for 30 min at 4°C with phycoerythrin (PE)-conjugated mAbs [CD25 to detect Tac-antigen/IL-2 receptor-α chain or Leu-3A to detect CD4 (Becton Dickinson)], washed, and fixed in 1% paraformaldehyde. Live cells were gated based on forward-scatter and side-scatter characteristics.
Determination of Endocytosis Rates.
Endocytosis rates were determined by using a FACS-based analysis as described (18). Transfected cells were stained with PE-conjugated antibody at 4°C as described above, except that azide was omitted. Before fixation, cells were incubated at 37°C for the indicated times, then stripped of surface antibody by brief incubation in saline (pH 2.0). To determine the maximal intensity of staining obtainable, aliquots of cells were taken post-staining and spared from acid-stripping; these samples were designated tx. Samples harvested at each time point (n) were designated tn. Samples designated t0 were subjected to acid-stripping, washing, and fixation without any incubation at 37°C. The amount of internalized label was determined from the mean PE-fluorescence of the green fluorescent protein (GFP)-positive cells in each sample and was expressed as [(tn − t0)/tx] × 100, the percent internalization.
Viral Production.
Proviral plasmids were used to transfect the T cell line CEM as described (7). The cultures were allowed to become chronically infected, at which time viral stocks were obtained by washing and resuspending the cells in fresh medium, then collecting the newly produced viral progeny over a 72-h period.
Determination of Viral Infectivity.
Particle infectivity was determined as described by using HeLa-CD4 cells as targets in a syncytium formation assay (32). The infectivities of the mutants were expressed relative to that of wild-type virus.
RESULTS
A Flow Cytometric Surface-Displacement Assay Categorizes the Endocytosis Signal in HIV-1 Nef as Dileucine-Based.
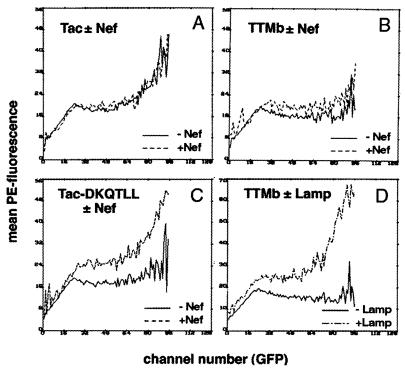
To determine whether the interaction of Nef with the cellular sorting machinery occurs through tyrosine- or dileucine-based motifs, we used a previously described endocytosis competition assay that can functionally categorize these signals (30). This approach is based on the observation that transmembrane proteins with functionally similar signals inhibit each others endocytosis to a much greater extent than proteins with functionally dissimilar signals. To categorize a suspected but unidentified sorting signal in a test protein, the ability of that protein to displace indicator proteins containing known signals to the cell surface can be measured by flow cytometry. Although Nef is a peripheral rather than a transmembrane protein, we reasoned that it might function in such an assay because its intracellular distribution overlaps that of clathrin and adaptor protein complexes (16, 17). Coexpression of HIV-1 Nef was tested for its effects on the surface expression of transmembrane proteins whose extracellular and transmembrane domains are identical (corresponding to those of the IL-2 receptor α chain, Tac) but whose cytoplasmic domains contain either a dileucine-based motif (Tac-DKQTLL), a tyrosine-based motif (TTMb), or no known motif (Tac) (30). Cells of the human kidney line 293 were transfected with plasmids expressing one of each of these proteins, with or without cotransfection of a plasmid expressing Nef as a competitor. A plasmid expressing the GFP was included to allow assessment of transfection efficiency. The expression of the Tac-based proteins at the cell surface was monitored by flow cytometry by using a PE-conjugated antibody to Tac (anti-CD25).
The surface expression of the Tac-based proteins was analyzed with respect to transfection efficiency by determining the mean PE fluorescence for each GFP channel number in each transfection experiment. The mean surface level of native Tac (PE fluorescence) increased with transfection efficiency (GFP fluorescence) (Fig. 1A). In contrast, Tac-DKQTLL and TTMb, proteins that are readily sorted away from the cell membrane, maintained low levels of surface expression over a broad range of transfection efficiencies (Fig. 1 B and C). Coexpression of Nef caused an increase in the surface expression of Tac-DKQTLL, the indicator protein that contains a dileucine-based motif (Fig. 1C). This effect was most evident at the highest GFP channel numbers. In contrast, coexpression of Nef had only a modest effect on the surface expression of TTMb, the indicator protein that contains a tyrosine-based motif, and no effect on the surface expression of native Tac, the indicator protein that contains no known endocytosis motif (Fig. 1 A and B). To confirm the inability of Nef to affect the surface expression of TTMb as a true negative, cotransfection of a plasmid encoding the transmembrane protein Lamp1, which contains a tyrosine-based motif, increased the surface expression of TTMb (Fig. 1D). The increase in surface expression of Tac-DKQTLL induced by Nef and of TTMb induced by Lamp1 were dose-dependent effects (data not shown). In the presence of maximal amounts of Nef expression plasmid (15 μg) the mean PE fluorescence (Tac-DKQTLL expression) for all GFP-positive cells increased by 2.7-fold (data not shown). The ability of HIV-1 Nef to increase the surface expression of Tac-DKQTLL together with its inability to increase the surface expression of TTMb, suggested that Nef contains a functional dileucine-based sorting motif but lacks a functional tyrosine-based motif.
Figure 1.
Effect of Nef on the surface expression of indicator proteins containing dileucine- or tyrosine-based sorting motifs. 293 cells were transfected with plasmids encoding the transmembrane indicator proteins Tac, IL-2 receptor-α chain (0.25 μg) (A); TTMb, a chimera containing the extracellular and transmembrane domains of Tac fused to a cytoplasmic domain containing a tyrosine-based motif (2.5 μg) (B and D); or Tac-DKQTLL, a fusion protein in which the dileucine-based motif DKQTLL is appended to the C terminus of the Tac cytoplasmic domain (2 μg) (C). A plasmid encoding the GFP (0.5 μg) was included as a transfection marker. Cells were stained with anti-CD25 (which recognizes Tac) conjugated to PE and analyzed by flow cytometry. The mean PE fluorescence (surface level of Tac) for each GFP channel number (transfection efficiency) is plotted for 293 cells transfected with each of the Tac indicator proteins with and without competitors. +Nef cells were transfected with 15 μg of Nef expression vector. +Lamp cells were transfected with 15 μg of Lamp1 expression vector.
A Hexameric Sequence Within the Solvent-Exposed, Unstructured, C-Terminal Loop of Nef Is a Functional Dileucine-Based Endocytosis Motif.
Examination of the sequence of Nef used in these experiments revealed a potential dileucine-based endocytosis motif, ENTSLL, in the solvent exposed, unstructured loop near the C-terminus of the protein. Comparison of primate lentiviral sequences derived from diverse isolates of HIV-1, HIV-2, and simian immunodeficiency virus revealed the consensus sequence E/DXXXLφ at this location (33). This consensus matches closely that of dileucine-based sorting motifs derived from a variety of cellular transmembrane proteins (29).
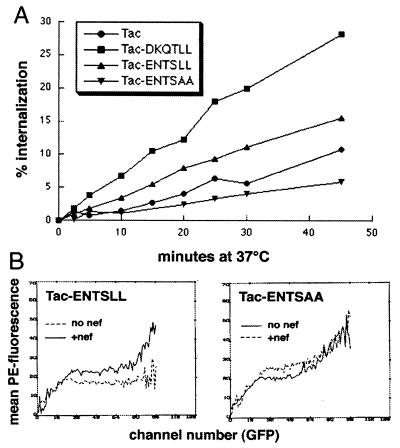
A characteristic of dileucine-based motifs is their ability to function as endocytosis signals when positioned at or near the C termini of heterologous proteins that are otherwise well expressed at the cell surface (28, 29). To determine whether the putative dileucine motif of Nef could function as an endocytosis signal, a vector was constructed to express a fusion protein in which the peptide sequence ENTSLL was appended to the cytoplasmic tail of IL-2 receptor α chain (pTac-ENTSLL). As a control, the vector pTac-ENTSAA was also constructed. The endocytosis rates of the Tac fusion molecules expressed by these vectors were compared with the rates of Tac and Tac-DKQTLL (Fig. 2A). The rate of endocytosis of Tac-DKQTLL was 2.8-fold greater than the rate of endocytosis of native Tac. Although the rate of endocytosis of Tac-ENTSLL was only 1.6-fold greater than that of native Tac, it was 2.8-fold greater than the rate of endocytosis of the matched control, Tac-ENTSAA. The efficiencies of surface expression observed for the Tac fusion proteins were inversely related to their relative rates of endocytosis (data not shown). These data demonstrated that the hexameric sequence ENTSLL of HIV-1 Nef is able to enhance the rate of endocytosis of a heterologous protein in a dileucine-dependent fashion.
Figure 2.
The Nef sequence ENTSLL functions as an endocytosis signal. (A) Endocytosis rates of Tac-based indicator proteins. Tac and Tac-DKQTLL are described in the legend of Fig. 1. Tac-ENTSLL contains the putative dileucine-motif in Nef appended to the C terminus of Tac. Tac-ENTSAA is identical to Tac-ENTSLL except that the leucines have been replaced with alanines. 293 cells were transfected with plasmids encoding Tac (5 μg), Tac-DKQTLL (10 μg), Tac-ENTSLL (6 μg), or Tac-ENTSAA (4 μg) and with a GFP-expression plasmid (0.5 μg), then stained with PE-conjugated anti-CD25 and incubated at 37°C for the indicated times before removal of label associated with the cell surface by acid wash and analysis by flow cytometry. The percentage of internalized label at each time point was calculated as described. The following slope values were calculated by using linear regression analysis: Tac, 0.23; Tac-DKQTLL, 0.64; Tac-ENTSLL, 0.36; Tac-ENTSAA, 0.13. (B) Effect of Nef on the surface expression of Tac-ENTSLL and Tac-ENTSAA. The mean PE fluorescence (Tac surface level) for each GFP channel number (transfection efficiency) is plotted for 293 cells transfected with Tac-ENTSLL and Tac-ENTSAA. +Nef cells were transfected with 15 μg of Nef expression vector. The amounts of indicator plasmids were as follows: Tac-ENTSLL, 0.5 μg; and Tac-ENTSAA, 0.1 μg.
To establish further the functional similarity of the ENTSLL and DKQTLL sequences, the effects of Nef on the surface expression of Tac-ENTSLL and Tac-ENTSAA were measured (Fig. 2B). Nef increased the efficiency with which Tac-ENTSLL was expressed at the cell surface but had little effect on the expression of Tac-ENTSAA. These observations indicated that intact Nef displaces to the cell surface a fusion protein containing a dileucine motif which is exactly homologous to that within its own primary protein structure.
Residues Within the Putative Endocytosis-Signal Are Required for Nef-Mediated Displacement of Transmembrane Proteins That Contain Dileucine Motifs to the Cell Surface.
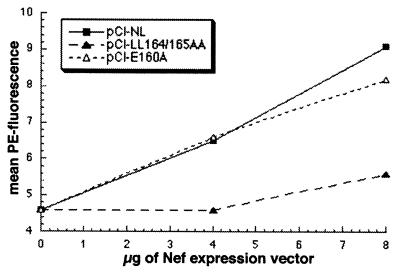
To determine whether the sequence ENTSLL is a functional sorting signal in the context of native Nef protein, mutations in this sequence were analyzed for their effects on Nef-mediated displacement of the fusion protein Tac-DKQTLL to the cell surface (Fig. 3). Vectors were constructed that express Nef proteins containing alanine substitution of the consecutive leucine residues (pCI-LL164/165AA) or alanine substitution of the glutamic acid residue (pCI-E160A). The effects of these mutations could be anticipated to some extent based on previous mutational analyses of dileucine-motifs in cellular transmembrane proteins (28, 29). The only absolute requirement for the function of these motifs is the first of the two consecutive leucines. The conserved acidic residue at position −4 relative to this leucine is variably important to endocytosis (29).
Figure 3.
The effect of mutations in the ENTSLL sequence of Nef on surface displacement of Tac-DKQTLL. 293 cells were transfected with a plasmid encoding Tac-DKQTLL (1 μg), a GFP-expression plasmid (0.5 μg), and the indicated amounts of the Nef expression plasmids. Transfected cells were analyzed by flow cytometry. The mean PE fluorescence (Tac surface levels) of GFP-positive cells is graphed versus the amount of Nef-expression plasmid. pCI-NL encodes wild-type Nef. pCI-LL164/165AA encodes a mutant in which the leucine residues within the sequence ENTSLL (residues 164 and 165 of HIV-1 NefNL4-3) are replaced with alanine residues. pCI-E160A encodes a mutant in which the glutamic acid residue within the sequence ENTSLL (residue 160) is replaced by an alanine residue.
As predicted by the hypothesis that the ENTSLL sequence is a functional sorting-signal in native Nef, the mutant Nef-LL164/165AA was unable to displace Tac-DKQTLL to the cell surface (Fig. 3). In contrast, the mutant Nef-E160A was almost equivalent to wild type in its ability to displace Tac-DKQTLL to the cell surface. Similar experiments were performed by using Tac-ENTSLL as the dileucine-containing indicator (data not shown). In general, these experiments yielded results similar to those obtained by using Tac-DKQTLL. Western blot analysis confirmed that comparable levels of Nef were expressed in these experiments (data not shown). These data indicated that the sequence ENTSLL in the C-terminal loop of Nef contains determinants important for the ability of native Nef to compete for access to the endocytic machinery. In particular, the dileucine residues of the ENTSLL sequence are required for the ability of Nef to displace transmembrane proteins that contain dileucine-based signals to the cell surface.
Residues Within the Putative Dileucine-Based Endocytosis-Motif of Nef Are Required for CD4 Down-Regulation.
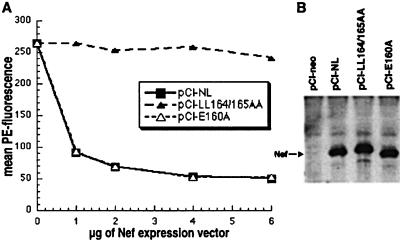
Current models suggest that Nef may physically connect CD4 to the endocytic machinery, causing an enhanced rate of endocytosis of CD4 from the cell surface (15, 18). Implicit in this hypothesis is the presence of a domain in Nef that is required for interaction with the endocytic machinery and, consequently, for down-regulation of CD4. To determine whether the dileucine motif in the C-terminal loop of Nef was required for down-regulation of CD4, the Nef mutants of the sequence ENTSLL described above were tested in a 293 cell-based, CD4 down-regulation assay (Fig. 4A). The mutant Nef-LL164/165AA, which was unable to displace dileucine-containing proteins to the cell surface, was unable to down-regulate CD4 (Fig. 4A). In contrast, the mutant Nef-E160A was essentially wild type in its ability to down-regulate CD4. Western blot analysis indicated that the levels of expression of the Nef proteins during these experiments were comparable (Fig. 4B). These effects of mutations within the ENTSLL sequence on CD4 down-regulation correlated closely with the effects on surface displacement of dileucine-containing proteins. This correlation supports the hypothesis that this sequence is an essential component of the recognition surface by which CD4–Nef complexes are connected to the endocytic machinery.
Figure 4.
The effect of mutations in the ENTSLL sequence of Nef on Nef-mediated down-regulation of CD4. (A) CD4 down-regulation. 293 cells were transfected with a CD4 expression plasmid (1 μg), a GFP-expression plasmid (0.5 μg), and the indicated amounts of the Nef-expression plasmids. Cells were stained with PE-conjugated anti-CD4 and analyzed by flow cytometry. The mean PE fluorescence (CD4 surface levels) of GFP-positive cells is graphed versus the amount of Nef expression plasmid. (B) Western blot analysis of Nef expression. Cells (5 × 104) analyzed in A and transfected with 2 μg of each Nef expression vector were lysed, separated electrophoretically, and analyzed as described (32).
Residues Within the Putative Dileucine-Based Endocytosis Motif of Nef Are Required for Optimal Viral Infectivity.
The enhancement by HIV-1 Nef of the infectivity of the cell-free virion and of viral replication appears causally separate from its ability to down-regulate CD4 (5, 7, 34–38). Nevertheless, we have observed a considerable overlap in the genetic determinants of CD4 down-regulation and enhancement of viral infectivity (unpublished data). Consequently, we hypothesized that these two phenomena might be related by an underlying property of Nef: the ability to influence the sorting of transmembrane proteins. This hypothesis predicts that mutations in the endocytosis motif of Nef will affect not only CD4 down-regulation but also viral infectivity and replication.
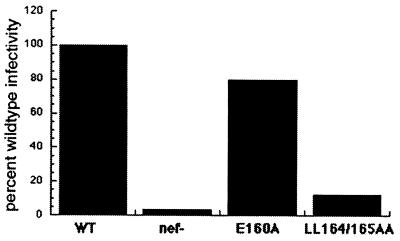
To determine whether the dileucine-based endocytosis motif in the C-terminal, solvent-exposed loop of Nef was important for enhancement of viral infectivity and replication, mutations within the ENTSLL sequence were created within the context of the complete viral genome. Viral stocks were produced as described and used to infect adherent, CD4-positive HeLa cells to determine the efficiency of formation of infectious centers as a measure of viral infectivity. The infectivities of cell-free virions produced by wild-type and nef-negative genomes were compared with those of virions produced by genomes containing the LL164/165AA or E160A mutations (Fig. 5). The mutation LL164/165AA reduced the infectivity of cell-free virus to levels only slightly above that of the nef-negative control. In contrast, the infectivity of the E160A mutant was almost equivalent to that of wild-type virus. The relative rates of replication of these viruses in primary cultures of human lymphoblasts showed a similar hierarchy (data not shown). These virologic effects of mutations within the ENTSLL sequence correlated closely with the effects of these mutations on Nef-mediated displacement of dileucine-containing transmembrane proteins to the cell surface and on Nef-mediated down-regulation of CD4. These data support the hypothesis that the ability of Nef to influence the sorting of transmembrane proteins is highly correlated with and may be the underlying cause of a variety of its phenotypic effects.
Figure 5.
The effect of mutations in the ENTSLL sequence of Nef on Nef-mediated enhancement of viral infectivity. The relative infectivities of cell-free virions produced from the indicated genomes were determined by using an infectious center assay in which CD4-positive HeLa cells were used as targets. The infectivities of the mutant virions were determined as described and are expressed relative to the wild type. WT, wild-type HIV-1NL4-3; nef−, nef-negative mutant containing two premature termination codons in the 5′ terminus of the nef-ORF (7); E160A, mutant encoding alanine substitution of E160; LL164/165AA, mutant encoding alanine substitutions of L164A and L165A.
DISCUSSION
By using a competition assay that functionally discriminates dileucine-based from tyrosine-based sorting signals, we have categorized the motif through which HIV-1 Nef interacts with the cellular endocytic machinery as dileucine-based. Inspection of the predicted amino acid sequences of primate lentiviruses revealed a conserved hexapeptide sequence (E/DXXXLφ) within the C-terminal, solvent-exposed, flexible loop of Nef protein which conforms to the consensus sequence of the dileucine-based sorting signals found in cellular transmembrane proteins. The corresponding sequence within NefNL4-3, ENTSLL, functioned as an endocytosis signal in the context of a heterologous protein and, in the context of intact Nef, was required for all Nef-associated functions tested including the ability to displace dileucine-containing transmembrane proteins to the cell surface, the ability to down-regulate CD4, and the ability to enhance viral infectivity and replication.
These experiments demonstrate use of the surface-displacement assay described by Marks et al. (30) to functionally categorize an endocytosis signal in a test protein. These investigators described the ability of transmembrane proteins containing similar sorting signals (tyrosine-based or dileucine-based) to inhibit each others endocytosis, causing displacement of the indicator protein to a “default” destination: the cell surface. By measuring the effect of Nef on the surface expression of the previously characterized indicator proteins Tac-DQKTLL (a fusion protein containing a dileucine sorting motif) and TTMb (a fusion protein containing a tyrosine sorting motif), the Nef protein was characterized functionally as containing a dileucine-like motif.
Sequence inspection, followed by mutational analyses, indicated that the dileucine-motif predicted from the surface-displacement assays was located in the central region of an unstructured, solvent-exposed, 30-amino acid residue loop near the C terminus of Nef protein. Our results show an example of a dileucine-based sorting motif described in a nontransmembrane protein; Nef is a peripheral membrane protein whose presumed association with the cytoplasmic leaflet requires N-terminal myristoylation (39). Nevertheless, the primary sequence, the genetic determinants of function, and the structural context of the dileucine motif in Nef are highly analogous to those of cellular, transmembrane proteins. Comparison of divergent isolates of HIV-1, HIV-2, and SIV revealed the consensus sequence E/DXXXLφ at this location in Nef (33). This is precisely the consensus sequence of the dileucine motifs described in cellular proteins (29). As in cellular dileucine motifs, the LL sequence was required in Nef for endocytic function. As in some but not all cellular sequences (29), the acidic residue at position −4 relative to the first of the consecutive leucines was dispensable for endocytic function. In some cellular proteins such as the invariant chain Iip31, this residue, though dispensable for endocytosis, appears required for the formation of large endosomal vesicles (29). Interestingly, this morphologic phenotype has been described in cells constitutively expressing Nef protein (40), and it will be of interest to determine if this phenotype requires the acidic residue in the Nef dileucine motif. Structurally, the dileucine motifs of cellular proteins are typically located near the extreme ends of the proteins’ cytoplasmic domains. NMR spectroscopy indicates that the dileucine motif within the cytoplasmic domain of the lysosomal integral membrane protein LIMPII exists within a random coil structure (41). The location of the Nef dileucine motif within an unfolded, solvent-exposed loop near the C terminus is consistent with this general structural description.
The location of the Nef dileucine motif within this solvent-exposed loop places it in a potentially ideal position for interaction with a protein ligand, presumably a component of the cellular endocytic machinery such as an adaptor complex protein. In this regard, direct interactions have been demonstrated between dileucine motifs and adaptor complex proteins of AP-1, AP-2, and AP-3 (42–44). Although in vitro peptide cross-linking studies have suggested that dileucine motifs are preferentially bound by the β-subunit of AP-1 complexes (42), yeast two-hybrid assays have suggested a direct interaction between the μ-subunit of AP-1 with HIV-1 Nef (17, 20). The interaction with μ1 was roughly mapped to the C-terminal solvent-exposed loop; however, the loop contains no sequences reminiscent of the tyrosine motifs known to interact directly with μ-subunits (17, 45). Together with the findings presented here, these data suggest the hypothesis that the dileucine motif in Nef directly interacts with the μ-subunit of adaptor complexes. In contrast, a recent analysis of HIV-2 and simian immunodeficiency virus Nef identified potential tyrosine motifs in the N terminus that appear to mediate an interaction with the μ-subunits of AP-1 and AP-2 (20). However, these tyrosines are not present in HIV-1 Nef, and mutation of these residues did not fully abrogate CD4 down-regulation (20). Furthermore, our data suggest that HIV-1 Nef does not interact functionally with the tyrosine-based sorting pathway.
Although the consecutive leucines in the ENTSLL sequence were required for Nef-mediated down-regulation of CD4, additional nearby residues within Nef appear required for this function (13, 46, 47). Of particular interest are charged residues located at the extreme C terminus of the flexible loop; these are required for both colocalization of Nef with adaptor complexes and for Nef-mediated CD4 down-regulation (16). These residues have recently been demonstrated to interact with a vacuolar ATPase involved in the acidification of endosomes and lysosomes (19). Thus, although we propose that the dileucine motif is required for the interaction of Nef with the endocytic machinery and consequently for CD4 down-regulation, it is almost certainly insufficient for these effects and may act cooperatively with other sequences in the C-terminal loop.
The ability of Nef to increase the surface expression of dileucine motif-containing indicator proteins such as Tac-DKQTLL seems paradoxical in view of its ability to decrease the surface expression of CD4, a transmembrane protein that itself contains a weak dileucine-based motif (14). Indeed, the LL sequence in the cytoplasmic domain of CD4 is required for Nef-mediated CD4 down-regulation (14). This paradox is resolvable in view of the apparent requirement for the LL sequence in CD4 for the binding interaction of CD4 and Nef (21, 22). Thus, by binding to the cytoplasmic domain of CD4, Nef may essentially cover an inherently weak endocytosis signal and replace it with a more potent signal from within its own structure, allowing relatively efficient endocytosis of CD4–Nef complexes. Alternatively, by recruiting CD4 to adaptor complexes, Nef may facilitate a direct interaction between the dileucine motif of CD4 and a component of the endocytic machinery.
The dileucine motif in Nef is important not only for down-regulation of CD4 but also for Nef-mediated enhancement of viral infectivity and replication. However, CD4 down-regulation does not appear to be the cause of Nef’s virologic effects; Nef enhances the infectivity of HIV-1 virions even when produced from CD4-negative cells (5, 7, 38). In addition, the LL164/165AA viral mutant is defective in infectivity even when produced from cells whose CD4 cannot respond to Nef (data not shown). What, then, may be the connection between the sorting motif in Nef and Nef’s virologic properties? Notably, Nef enhances infectivity before viral gene expression in the newly infected target cell. Indeed, Nef modifies the virion during its production such that it establishes viral DNA in the target cell more efficiently (5, 32, 36, 38). The nature of this virion modification and the mechanism of enhanced viral DNA synthesis are unknown. We propose that the influence of Nef on the sorting of transmembrane proteins may alter the composition of the viral as well as the cellular membrane in a manner that renders the virion more infectious. In this regard, it is interesting to consider not only the possibility of down-regulation of surface proteins such as CD4, but also the potential for upregulation of proteins in a manner analogous to the surface-displacement effects reported here. Although the mechanism of infectivity enhancement mediated by the Nef dileucine motif remains unclear, these data suggest that dysregulation of protein sorting may unify two seemingly distinct properties of Nef: down-regulation of CD4 and enhancement of viral infectivity.
Acknowledgments
We are indebted to Juan Bonifacino for providing the plasmids pCDM8-Tac, pTac-DKQTLL, pTTMb, and pCDM8-Lamp1. We also thank Phillip Dao, Nancy Keating, Michelle Lutz, Judy Nordberg, and Nanette Riggs for technical support; Darica Smith and Sharon Wilcox for administrative support; Celsa Spina for provision of anti-Nef antiserum; Didier Trono for provision of the plasmid pCMX-CD4; and Douglas Richman for support and encouragement. HT4-6C cells were obtained from the AIDS Research and Reagent program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and were provided by Bruce Chesebro. This work was supported by a grant from the National Institutes of Health (AI38201), the University of California, San Diego, Center for AIDS Research (AI36214), and the Research Center for AIDS and HIV Infection of the San Diego Veterans Affairs Medical Center.
ABBREVIATIONS
- Tac
IL-2 receptor-α chain
- φ
amino acid residue with bulky, hydrophobic side chain
- IL
interleukin
- PE
phycoerythrin
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 2.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, et al. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 3.Terwilliger E, Sodroski J G, Rosen C A, Haseltine W A. J Virol. 1986;60:754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ronde A, Klaver B, Keulen W, Smit L, Goudsmit J. Virology. 1992;187:391–395. doi: 10.1016/0042-6822(92)90772-h. [DOI] [PubMed] [Google Scholar]
- 5.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowers M Y, Spina C A, Kwoh T J, Fitch N J S, Richman D D, Guatelli J C. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy B, Kieny M-P, Reviere Y, La Peuch C, Dott K, Girand M, Montagnier L, Lecocq J-P. Nature (London) 1987;330:266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- 9.Garcia J V, Miller A D. Nature (London) 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard J-M. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 11.Luria S, Chambers I, Berg P. Proc Natl Acad Sci USA. 1991;88:5326–5330. doi: 10.1073/pnas.88.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 13.Iafrate A J, Bronson S, Skowronski J. EMBO J. 1997;16:101–112. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiken C, Konner J, Landau N R, Lenburg M, Trono D. Cell. 1994;76:853–854. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 15.Foti M, Mangasarian A, Piguet V, Lew D P, Krause K-H, Trono D, Carpentier J-L. J Cell Biol. 1997;139:37–47. doi: 10.1083/jcb.139.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J-M, Schwartz O. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 18.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J-L, Trono D. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 19.Lu X, Haifeng Y, Liu S-H, Brodsky F M, Peterlin B M. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 20.Piguet V, Chen Y-L, Mangasarian A, Foti M, Carpentier J-L, Trono D. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grzesiek S, Stahl S J, Wingfield P T, Bax A. Biochemistry. 1996;35:10256–10261. doi: 10.1021/bi9611164. [DOI] [PubMed] [Google Scholar]
- 22.Rossi F, Gallina A, Milanesi G. Virology. 1996;217:397–403. doi: 10.1006/viro.1996.0130. [DOI] [PubMed] [Google Scholar]
- 23.Harris M P, Neil J C. J Mol Biol. 1994;241:136–142. doi: 10.1006/jmbi.1994.1483. [DOI] [PubMed] [Google Scholar]
- 24.Lee C H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 25.Grzesiek S, Bax A, Clore G M, Groenborn A M, Kaufman J, Palmer I, Stahl S J, Wingfield P T. Nat Struct Biol. 1996;3:340–344. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- 26.Kirchhausen T, Bonifacino J S, Reizman H. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 27.Trowbridge I S, Collawn J F, Hopkins C R. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 28.Letourneur F, Klausner R D. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 29.Pond L, Kuhn L A, Teyton L, Schutze M-P, Tainer J A, Jackson M R, Peterson P A. J Biol Chem. 1995;270:19989–19997. doi: 10.1074/jbc.270.34.19989. [DOI] [PubMed] [Google Scholar]
- 30.Marks M S, Woodruff L, Ohno H, Bonifacino J S. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandori M W, Fitch N J S, Craig H M, Richman D D, Spina C A, Guatelli J C. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shugars D C, Smith M S, Glueck D H, Nantermet P V, Seillier-Moiseiwitsch F, Swanstrom R. J Virol. 1993;67:4639–4650. doi: 10.1128/jvi.67.8.4639-4650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saksela K, Cheng G, Baltimore D. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldsmith M A, Warmerdam M T, Atchison R E, Miller M D, Greene W C. J Virol. 1995;69:4112–4121. doi: 10.1128/jvi.69.7.4112-4121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chowers M Y, Pandori M W, Spina C A, Richman D D, Guatelli J C. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz O, Maréchal V, Danos O, Heard J-M. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aiken C, Trono D. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaminchik J, Bashan N, Itach A, Sarver N, Gorecki M, Panet A. J Virol. 1991;65:583–588. doi: 10.1128/jvi.65.2.583-588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanfridson A, Hester S, Doyle C. Proc Natl Acad Sci USA. 1997;94:873–878. doi: 10.1073/pnas.94.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandoval I V, Arredondo J J, Alcalde J, Noriega A G, Vandekerckhove J, Jimenez M A, Rico M. J Biol Chem. 1994;269:6622–6631. [PubMed] [Google Scholar]
- 42.Rapoport I, Chen Y C, Cupers P, Shoelson S E, Kirchhausen T. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heilker R, Manning-Krieg U, Zuber J-F, Spiess M. EMBO J. 1998;15:2893–2899. [PMC free article] [PubMed] [Google Scholar]
- 44.Honing S, Sandoval I V, von Figura K. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohno H, Stewart J, Fournier M C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 46.Aiken C, Krause L, Chen Y-L, Trono D. Virology. 1996;217:293–300. doi: 10.1006/viro.1996.0116. [DOI] [PubMed] [Google Scholar]
- 47.Hua J, Blair W, Truant R, Cullen B R. Virology. 1997;231:231–238. doi: 10.1006/viro.1997.8517. [DOI] [PubMed] [Google Scholar]