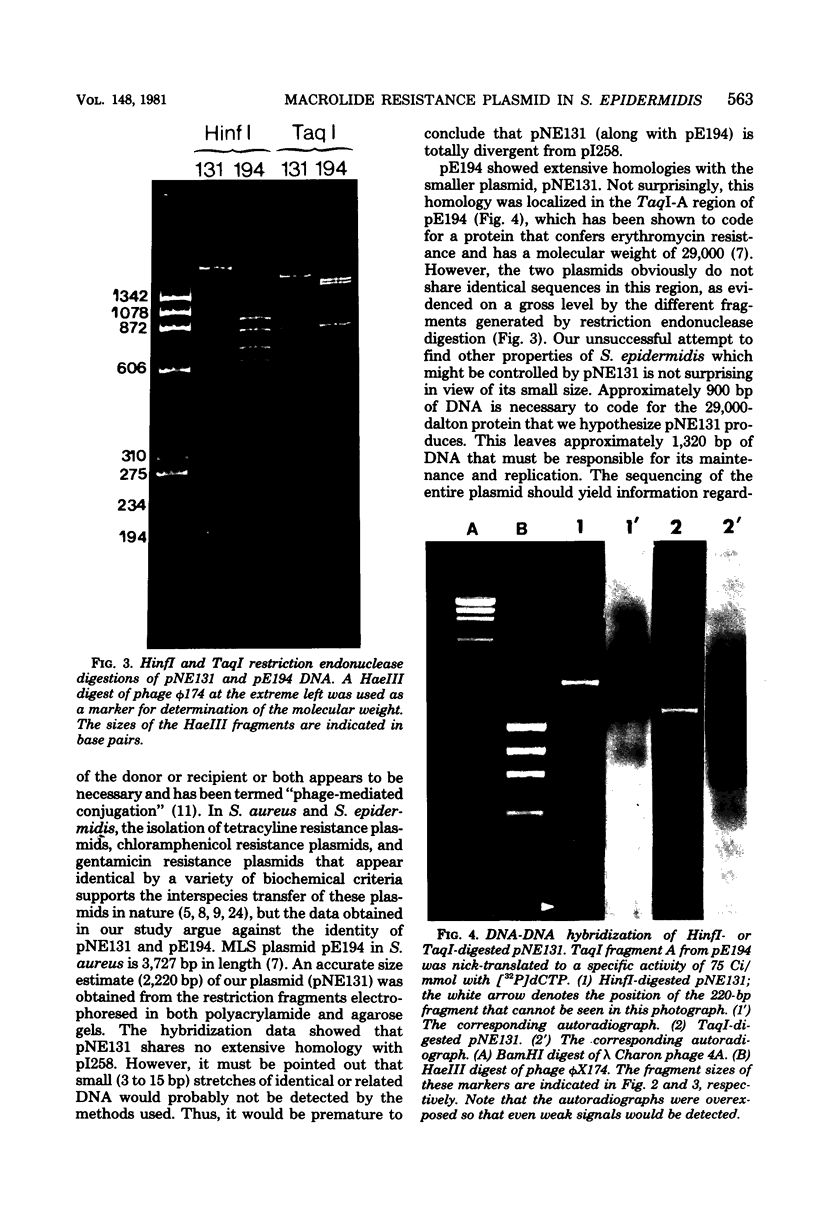
Abstract
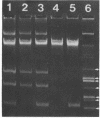
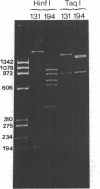
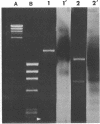
A strain of Staphylococcus epidermidis was transduced to erythromycin resistance, and all of the transductants exhibited the macrolide, lincosamide, streptogramin B resistance phenotype. Curing and antibiotic disk studies also indicated that these resistances were controlled by a single plasmid determinant and were constitutive. Agarose gel electrophoresis of plasmid deoxyribonucleic acid (DNA) from donor, cured, and transduced strains showed that a single plasmid was responsible. This plasmid, designated pNE131, was examined for sequence homology to two other plasmids, pE194 and p1258, from Staphylococcus aureus, which also code for erythromycin resistance. DNA from plasmids pNE131 and pE194 hybridized with one another, but no extensive homology to pI258 with either pNE131 or pE194 was found. Restriction endonuclease digests of pNE131 and pE194 showed no common fragments. However, sequence homology was localized to the nucleotides in pE194 that code for the 29,000-dalton protein responsible for erythromycin resistance. pNE131 was calculated to have 2,220 base pairs and is the smallest naturally occurring plasmid with a known function yet reported in S. epidermidis.
Full text
PDF

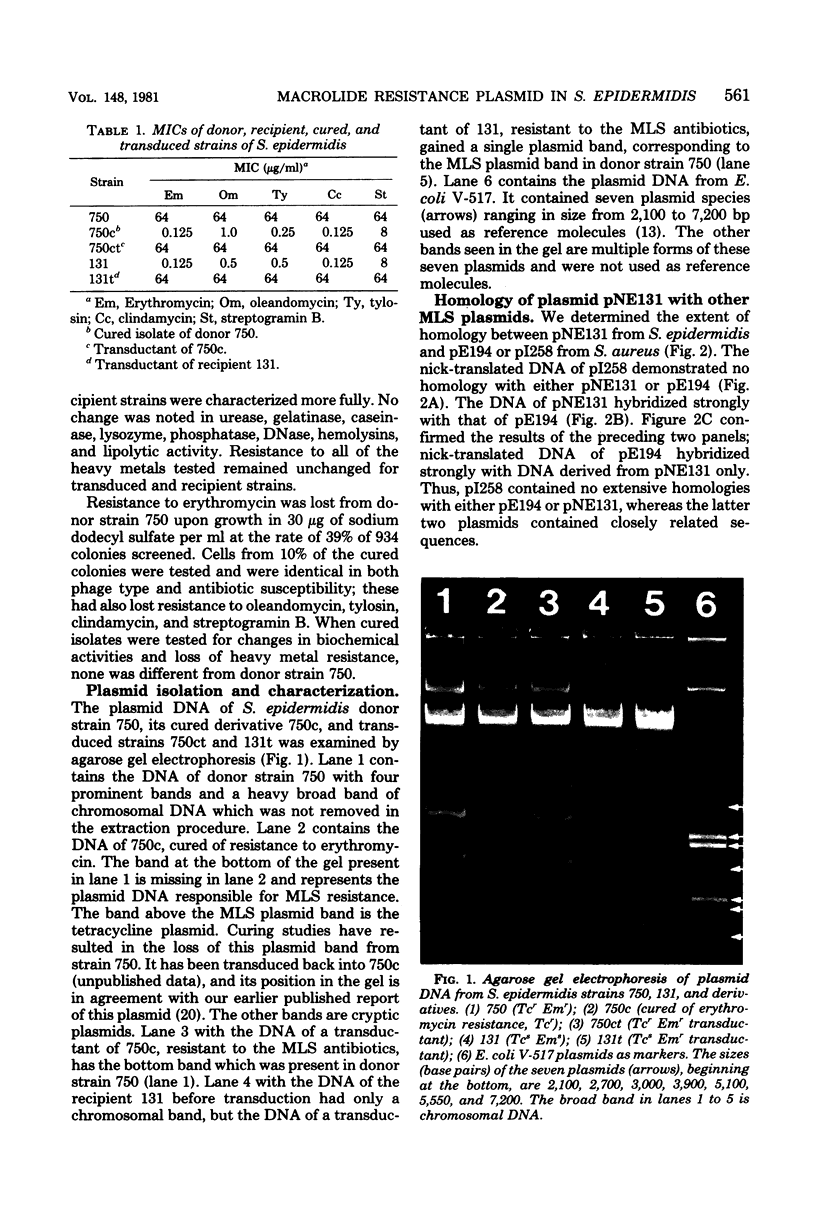


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastos M. C., Bonaldo M. C., Penido E. G. Constitutive erythromycin resistance plasmid in Staphylococcus aureus. J Gen Microbiol. 1980 Dec;121(2):513–516. doi: 10.1099/00221287-121-2-513. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Groves D. J. Interspecific relationships of antibiotic resistance in Staphylococcus sp.: isolation and comparison of plasmids determining tetracycline resistance in S. aureus and S. epidermidis. Can J Microbiol. 1979 Dec;25(12):1468–1475. doi: 10.1139/m79-227. [DOI] [PubMed] [Google Scholar]
- Hecht D. W., Parisi J. T. Characterization of the tobramycin-kanamycin-neomycin resistance plasmid in Staphylococcus epidermidis. J Antibiot (Tokyo) 1980 Aug;33(8):891–894. doi: 10.7164/antibiotics.33.891. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanescu S., Surdeanu M., Della Latta P., Novick R. Incompatibility and molecular relationships between small Staphylococcal plasmids carrying the same resistance marker. Plasmid. 1978 Sep;1(4):468–479. doi: 10.1016/0147-619x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Jaffe H. W., Sweeney H. M., Nathan C., Weinstein R. A., Kabins S. A., Cohen S. Identity and interspecific transfer of gentamicin-resistance plasmids in Staphylococcus aureus and Staphylococcus epidermidis. J Infect Dis. 1980 Jun;141(6):738–747. doi: 10.1093/infdis/141.6.738. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Terminal nucleotide sequences of Tn551, a transposon specifying erythromycin resistance in Staphylococcus aureus: homology with Tn3. Plasmid. 1980 Sep;4(2):148–154. doi: 10.1016/0147-619x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Evidence for two mechanisms of plasmid transfer in mixed cultures of Staphylococcus aureus. J Gen Microbiol. 1980 Aug;119(2):423–435. doi: 10.1099/00221287-119-2-423. [DOI] [PubMed] [Google Scholar]
- Lindberg M., Novick R. P. Plasmid-specific transformation in Staphylococcus aureus. J Bacteriol. 1973 Jul;115(1):139–145. doi: 10.1128/jb.115.1.139-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Males B. M., Rogers W. A., Jr, Parisi J. T. Virulence factors of biotypes of Staphylococcus epidermidis from clinical sources. J Clin Microbiol. 1975 Mar;1(3):256–261. doi: 10.1128/jcm.1.3.256-261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Schwesinger M. D., Gruss A. D., Swanson E. C., Pattee P. A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W. C., Jr, Parisi J. T., Totten P. A., Baldwin J. N. Transduction of penicillinase production in Staphylococcus epidermidis and nature of the genetic determinant. Can J Microbiol. 1979 Apr;25(4):508–511. doi: 10.1139/m79-074. [DOI] [PubMed] [Google Scholar]
- Parisi J. T., Hecht D. W. Plasmid profiles in epidemiologic studies of infections by Staphylococcus epidermidis. J Infect Dis. 1980 May;141(5):637–643. doi: 10.1093/infdis/141.5.637. [DOI] [PubMed] [Google Scholar]
- Parisi J. T., Talbot H. W., Jr Improved rapid plate method for the isolation of bacteriophages from lysogenic bacteria. Appl Microbiol. 1974 Sep;28(3):503–504. doi: 10.1128/am.28.3.503-504.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi J. T., Talbot H. W., Skahan J. M. Development of a phage typing set for Staphylococcus epidermidis in the United States. Zentralbl Bakteriol Orig A. 1978 Jul;241(1):60–67. [PubMed] [Google Scholar]
- Sjöström J. E., Löfdahl S., Philipson L. Transformation of Staphylococcus aureus by heterologous plasmids. Plasmid. 1979 Oct;2(4):529–535. doi: 10.1016/0147-619x(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W. Transfer of drug-resistance-plasmids in mixed cultures of Staphylococci. Zentralbl Bakteriol Orig A. 1977;237(2-3):147–159. [PubMed] [Google Scholar]