Abstract
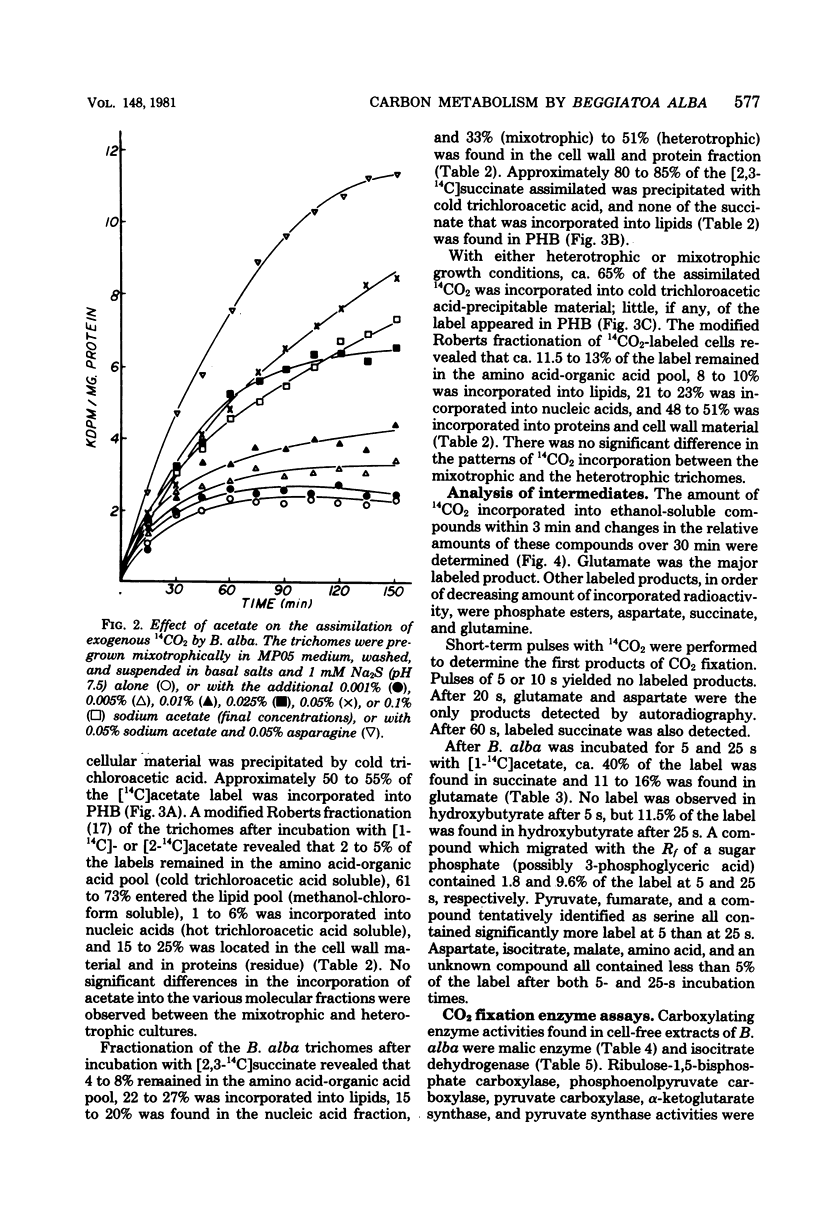
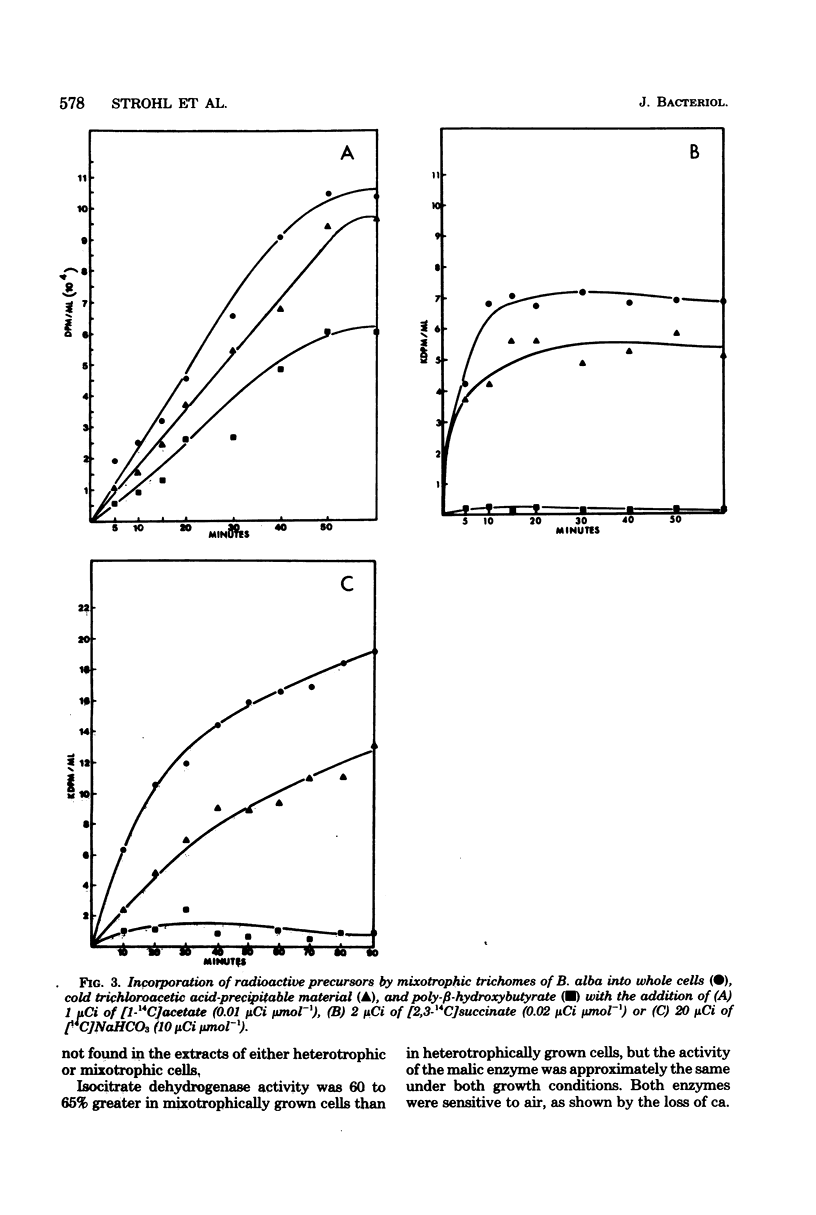
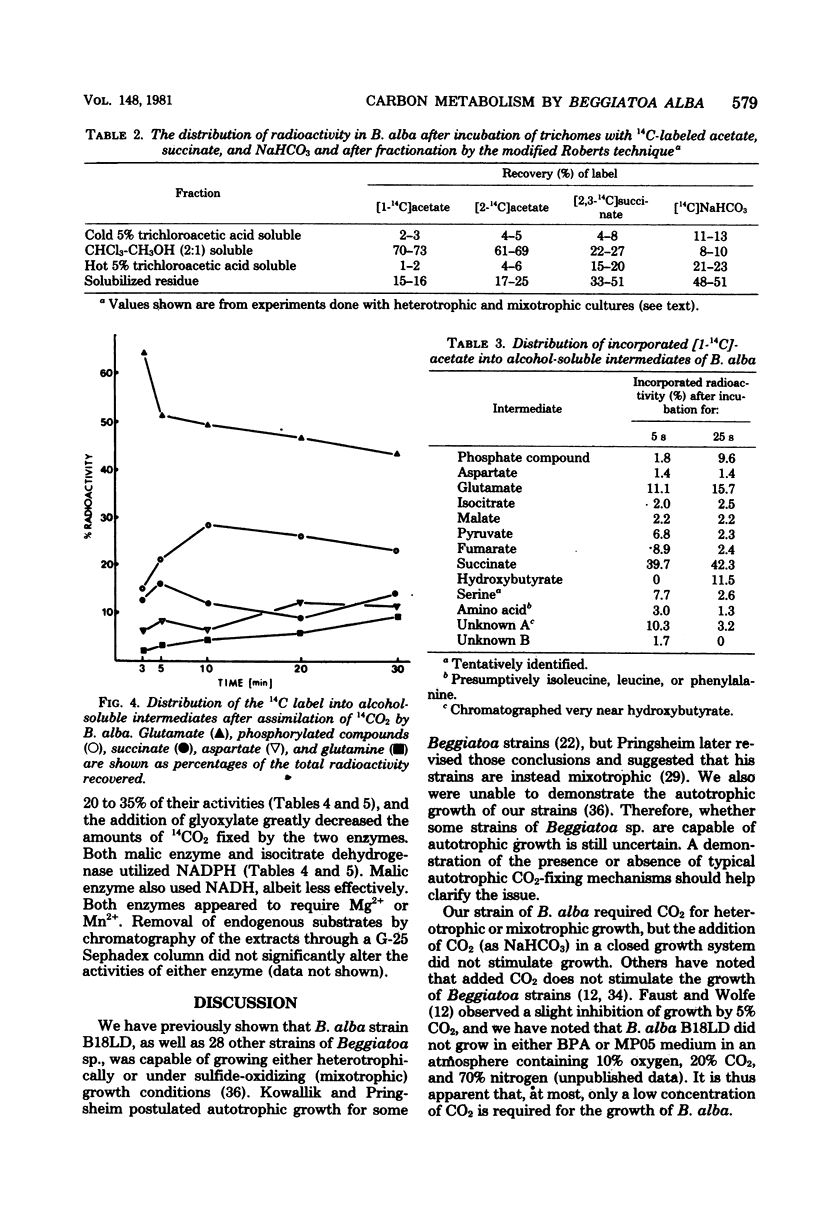
The assimilation and metabolism of CO2 and acetate by Beggiatoa alba strain B18LD was investigated. Although B. alba was shown to require CO2 for growth, the addition of excess CO2 (as NaHCO3) to the medium in a closed system did not stimulate growth. Approximately 24 to 31% of the methyl-labeled acetate and 38 to 46% of the carboxyl-labeled acetate were oxidized to 14CO2 by B. alba. The apparent Vmax values for combined assimilation and oxidation of [2-14C]acetate by B. alba were 126 to 202 nmol min−1 mg of protein−1 under differing growth conditions. The Vmax values for CO2 assimilation by heterotrophic and mixotrophic cells were 106 and 131 pmol min−1 mg of protein−1, respectively. The low Vmax values for CO2 assimilation, coupled with the high Vmax values for acetate oxidation, suggested that the required CO2 was endogenously produced from acetate. Moreover, exogenously supplied acetate was required by B. alba for the fixation of CO2. From 61 to 73% of the [14C]acetate assimilated by washed trichomes was incorporated into lipid. Fifty-five percent of the assimilated [2-14C]acetate was incorporated into poly-β-hydroxybutyric acid. This was consistent with chemical data showing that 56% of the heterotrophic cell dry weight was poly-β-hydroxybutyric acid. Succinate and CO2 were incorporated into cell wall material, proteins, lipids, nucleic acids, and amino and organic acids, but not into poly-β-hydroxybutyric acid. Glutamate and succinate were the major stable products after short-term [1-14C]acetate assimilation. Glutamate and aspartate were the first stable 14CO2 fixation products, whereas glutamate, a phosphorylated compound, succinate, and aspartate were the major stable 14CO2 fixation products over a 30-min period. The CO2 fixation enzymes isocitrate dehydrogenase (nicotinamide adenine dinucleotide phosphate; reversed) and malate dehydrogenase (nicotinamide adenine dinucleotide phosphate; decarboxylating) were found in cell-free extracts of both mixotrophically grown and heterotrophically grown cells. The data indicate that the typical autotrophic CO2 fixation mechanisms are absent from B. alba B18LD and that the CO2 and acetate metabolism pathways are probably linked.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BURTON S. D., MORITA R. Y. EFFECT OF CATALASE AND CULTURAL CONDITIONS ON GROWTH OF BEGGIATOA. J Bacteriol. 1964 Dec;88:1755–1761. doi: 10.1128/jb.88.6.1755-1761.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton S. D., Morita R. Y., Miller W. Utilization of acetate by Beggiatoa. J Bacteriol. 1966 Mar;91(3):1192–1200. doi: 10.1128/jb.91.3.1192-1200.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B., Arnon D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):928–934. doi: 10.1073/pnas.55.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAUST L., WOLFE R. S. Enrichment and cultivation of Beggiatoa alba. J Bacteriol. 1961 Jan;81:99–106. doi: 10.1128/jb.81.1.99-106.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK G. DIE BIOSYNTHESE DER POLY-BETA-HYDROXYBUTTERSAEURE DURCH KNALLGASBAKTERIEN. III. SYNTHESE AUS KOHLENDIOXYD. Arch Mikrobiol. 1964 Feb 21;47:236–250. [PubMed] [Google Scholar]
- Gehring U., Arnon D. I. Purification and properties of -ketoglutarate synthase from a photosynthetic bacterium. J Biol Chem. 1972 Nov 10;247(21):6963–6969. [PubMed] [Google Scholar]
- Güde H., Strohl W. R., Larkin J. M. Mixotrophic and heterotrophic growth of Beggiatoa alba in continuous culture. Arch Microbiol. 1981 Jul;129(5):357–360. doi: 10.1007/BF00406462. [DOI] [PubMed] [Google Scholar]
- Ihlenfeldt M. J., Gibson J. Acetate uptake by the unicellular cyanobacteria Synechococcus and Aphanocapsa. Arch Microbiol. 1977 Jun 20;113(3):231–241. doi: 10.1007/BF00492030. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Calder J. A., Van Baalen C., Plucker F. E., Parker P. L. Role of reduced exogenous organic compounds in the physiology of the blue-green bacteria (algae): photoheterotrophic growth of a "heterotrophic" blue-green bacterium. J Bacteriol. 1973 May;114(2):695–700. doi: 10.1128/jb.114.2.695-700.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. J., Abraham S. Assimilation and metabolism of exogenous organic compounds by the strict autotrophs Thiobacillus thioparus and Thiobacillus neapolitanus. J Bacteriol. 1969 Mar;97(3):1198–1208. doi: 10.1128/jb.97.3.1198-1208.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., PHIZACKERLEY P. J., SADLER J. R. The metabolism of C2 compounds in micro-organisms. 5. Biosynthesis of cell materials from acetate in Escherichia coli. Biochem J. 1960 Dec;77:438–445. doi: 10.1042/bj0770438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Myers W. F., Huang K. Y. Separation of intermediates of the citric acid cycle and related compounds by thin-layer chromatography. Anal Biochem. 1966 Nov;17(2):210–213. doi: 10.1016/0003-2697(66)90199-0. [DOI] [PubMed] [Google Scholar]
- Nishikido T., Izui K., Iwatani A., Katsuki H., Tanaka S. Inhibition of the carbon dioxide fixation of E. coli by the compounds related to TCA cycle. Biochem Biophys Res Commun. 1965 Oct 26;21(2):94–99. doi: 10.1016/0006-291x(65)90092-6. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. I. Ingibition of isocitrate lyase and isocitrate dehydrogenase by organic acids related to the TCA and glyoxylate cycles. J Biochem. 1968 Sep;64(3):355–363. doi: 10.1093/oxfordjournals.jbchem.a128902. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Pringsheim E. G. Die Mixotrophie von Beggiatoa. Arch Mikrobiol. 1967;59(1):247–254. [PubMed] [Google Scholar]
- SCOTEN H. L., STOKES J. L. Isolation and properties of Beggiatoa. Arch Mikrobiol. 1962;42:353–368. doi: 10.1007/BF00409071. [DOI] [PubMed] [Google Scholar]
- Sadler W. R., Stanier R. Y. THE FUNCTION OF ACETATE IN PHOTOSYNTHESIS BY GREEN BACTERIA. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1328–1334. doi: 10.1073/pnas.46.10.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. R., Larkin J. M. Enumeration, isolation, and characterization of beggiatoa from freshwater sediments. Appl Environ Microbiol. 1978 Nov;36(5):755–770. doi: 10.1128/aem.36.5.755-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Rupprecht E., Jungermann K. Glyoxylate inhibition of clostridial pyruvate synthase. FEBS Lett. 1970 Aug 31;9(5):271–273. doi: 10.1016/0014-5793(70)80374-x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., WILKINSON J. F. The isolation and estimation of the poly-beta-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol. 1958 Aug;19(1):198–209. doi: 10.1099/00221287-19-1-198. [DOI] [PubMed] [Google Scholar]



