Abstract
By using genetic analysis, the mutations of eight glutamine-requiring mutants isolated from Bacillus subtilis 168 were all shown to be linked to the thyA marker. A three-factor transduction analysis performed with one of the gln mutations indicated that the gene order in this region of the B. subtilis chromosome was gltA-thyA-gln. On the basis of recombination index values, two closely linked groups were identified. The mutations belonging to one group were assigned to the structural gene for glutamine synthetase, and those belonging to the other group might impair a regulatory locus. The residual glutamine synthetase activities and the cross-reacting materials of the mutants from both recombination groups supported these conclusions.
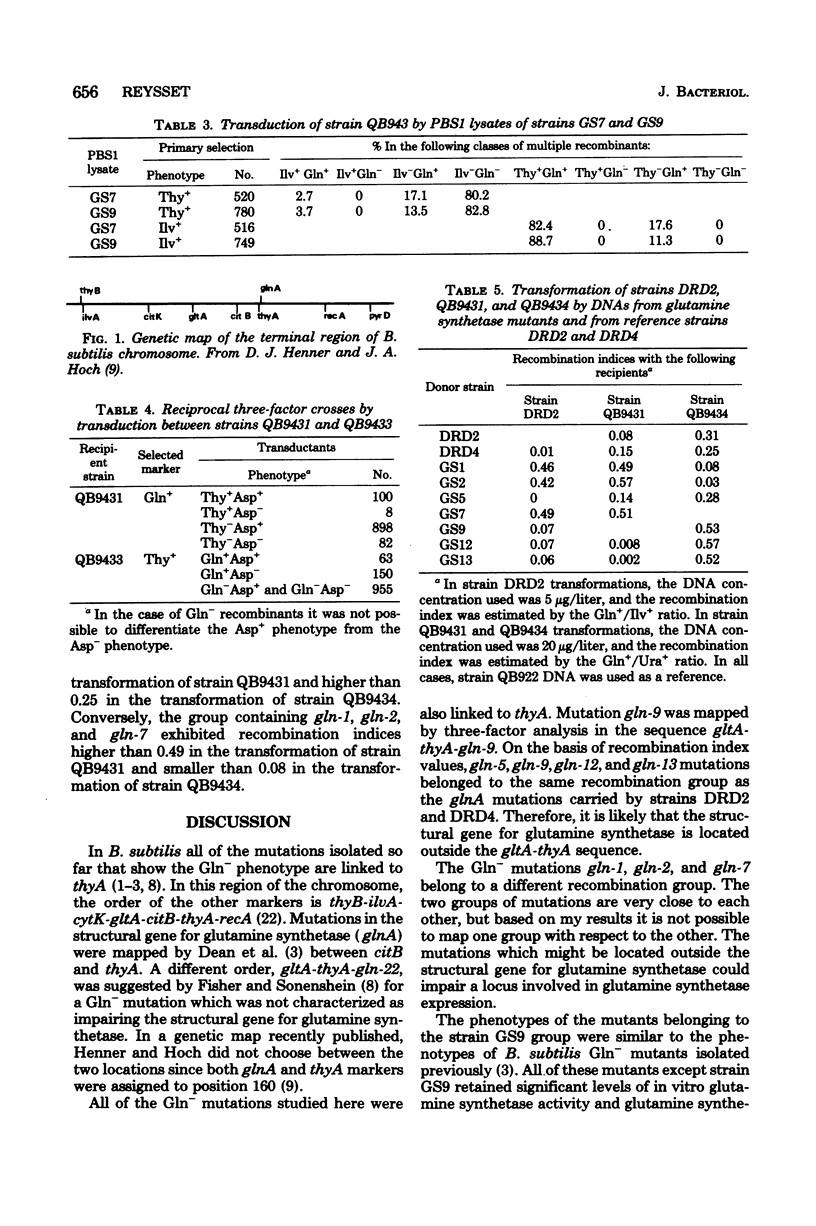
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bott K. F., Reysset G., Gregoire J., Islert D., Aubert J. P. Characterization of glutamine requiring mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1977 Dec 7;79(3):996–1003. doi: 10.1016/0006-291x(77)91208-6. [DOI] [PubMed] [Google Scholar]
- Dean D. R., Aronson A. I. Selection of Bacillus subtilis mutants impaired in ammonia assimilation. J Bacteriol. 1980 Feb;141(2):985–988. doi: 10.1128/jb.141.2.985-988.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Hoch J. A., Aronson A. I. Alteration of the Bacillus subtilis glutamine synthetase results in overproduction of the enzyme. J Bacteriol. 1977 Sep;131(3):981–987. doi: 10.1128/jb.131.3.981-987.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Ginsburg A., Yeh J., Shelton E., Stadtman E. R. Bacillus subtilis glutamine synthetase. Purification and physical characterization. J Biol Chem. 1970 Oct 25;245(20):5195–5205. [PubMed] [Google Scholar]
- Elmerich C. Le cycle du glutamate, point de départ du métabolisme de l'azote, chez Bacillus megaterium. Eur J Biochem. 1972 May 23;27(2):216–224. doi: 10.1111/j.1432-1033.1972.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Glutamine-requiring mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1977 Dec 7;79(3):987–995. doi: 10.1016/0006-291x(77)91207-4. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Hoch J. A. The Bacillus subtilis chromosome. Microbiol Rev. 1980 Mar;44(1):57–82. doi: 10.1128/mr.44.1.57-82.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Burton D., Garcia E., McCarter L., McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo J. M., Goldberg R. B. Regulation of nitrogen metabolism in glutamine auxotrophs of Klebsiella pneumoniae. J Bacteriol. 1980 Apr;142(1):99–110. doi: 10.1128/jb.142.1.99-110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers J. L., Tempest D. W., Brown C. M. 'Glutamine(amide):2-oxoglutarate amino transferase oxido-reductase (NADP); an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol. 1970 Dec;64(2):187–194. doi: 10.1099/00221287-64-2-187. [DOI] [PubMed] [Google Scholar]
- Pahel G., Tyler B. A new glnA-linked regulatory gene for glutamine synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4544–4548. doi: 10.1073/pnas.76.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reysset G., Aubert J. P. Relationship between sporulation and mutations impairing glutamine synthetase in Bacillus megaterium. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1237–1241. doi: 10.1016/s0006-291x(75)80362-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA A., FREESE E. ENZYMATIC PROPERTIES OF ALANINE DEHYDROGENASE OF BACILLUS SUBTILIS. Biochim Biophys Acta. 1965 Feb 22;96:248–262. [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



