Abstract
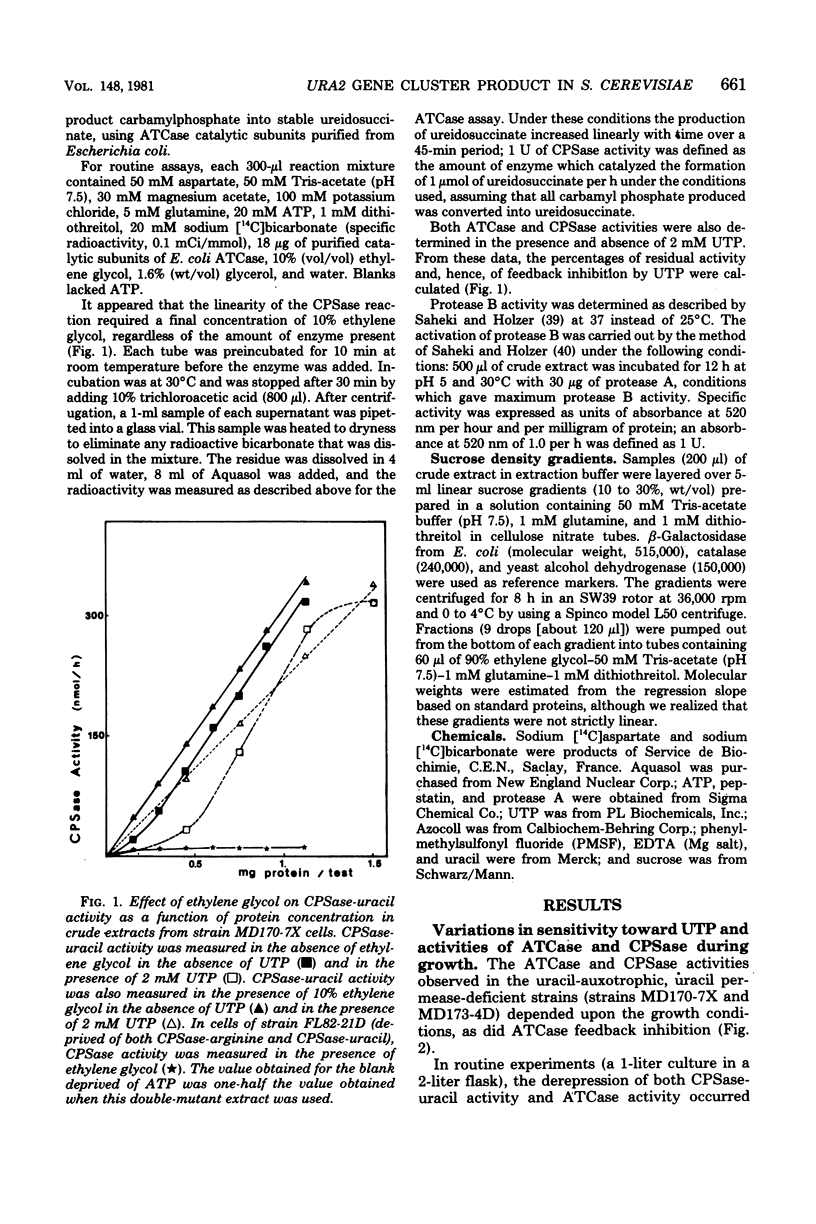
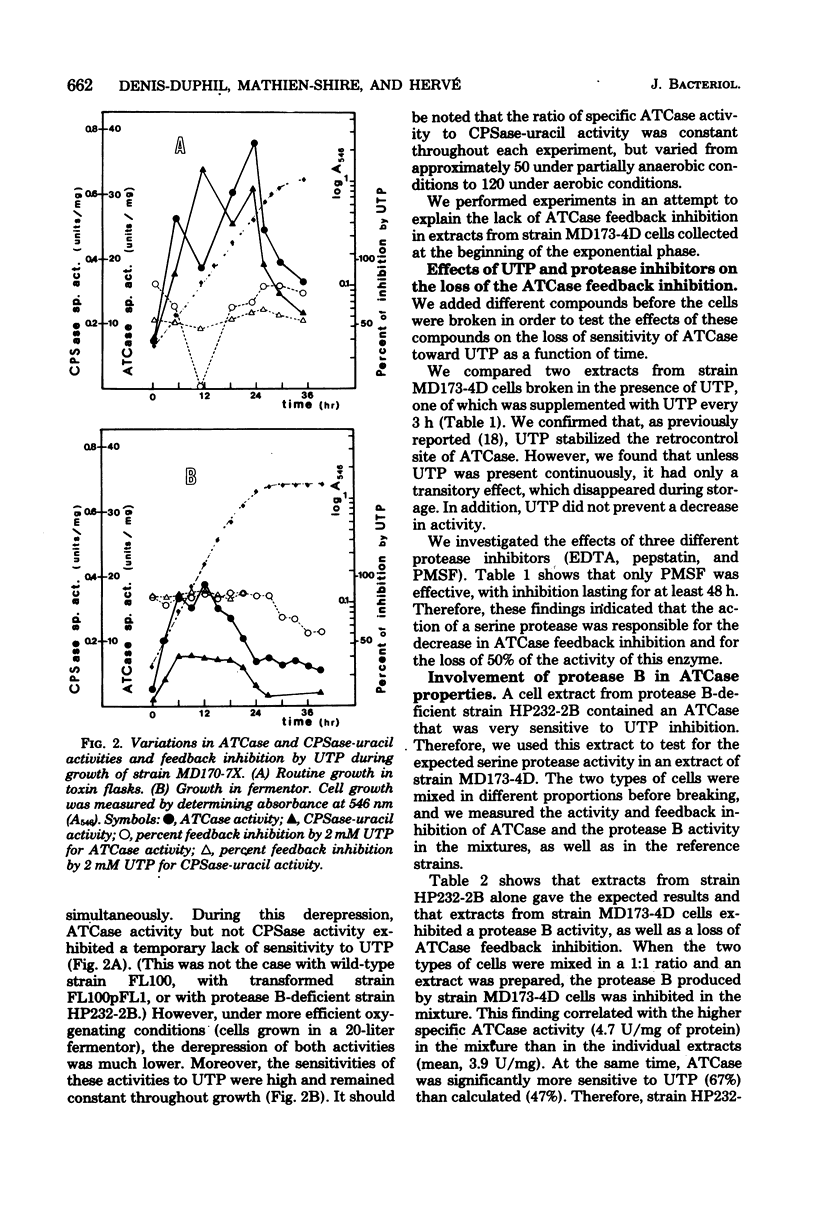
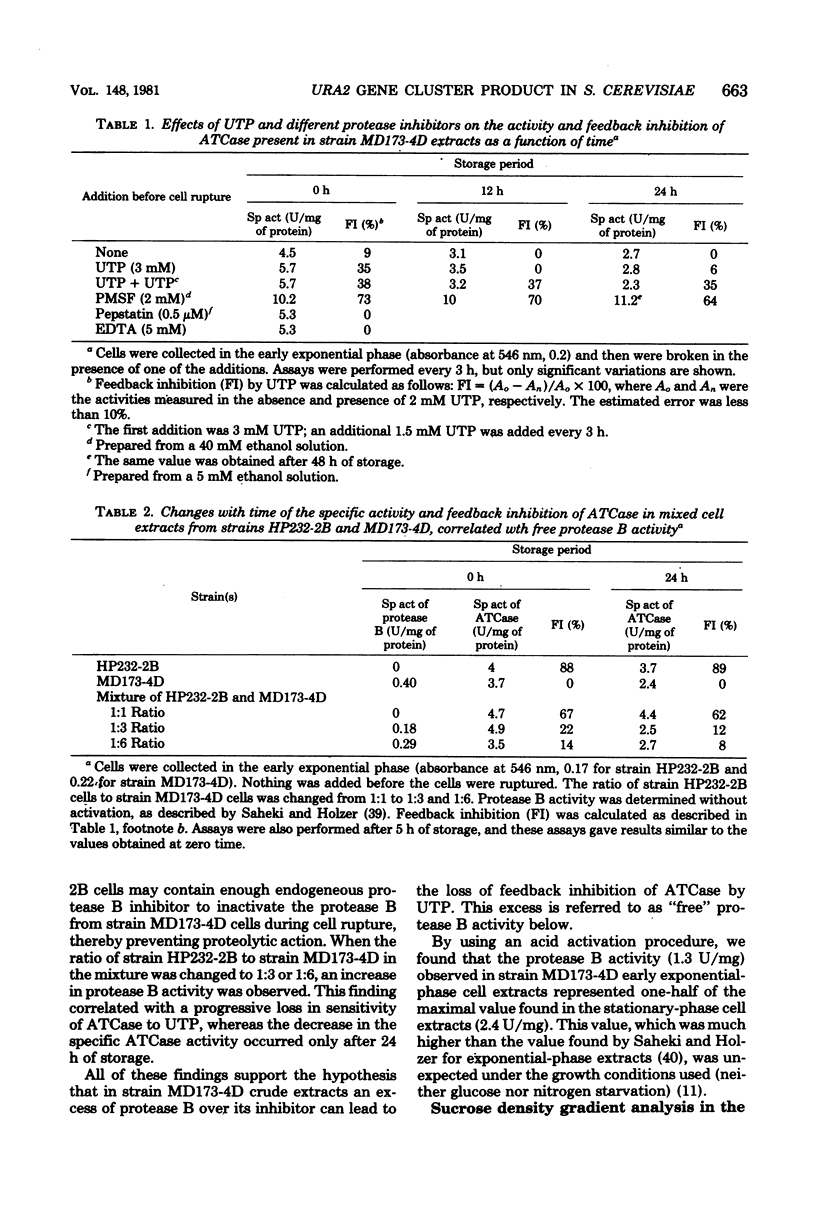
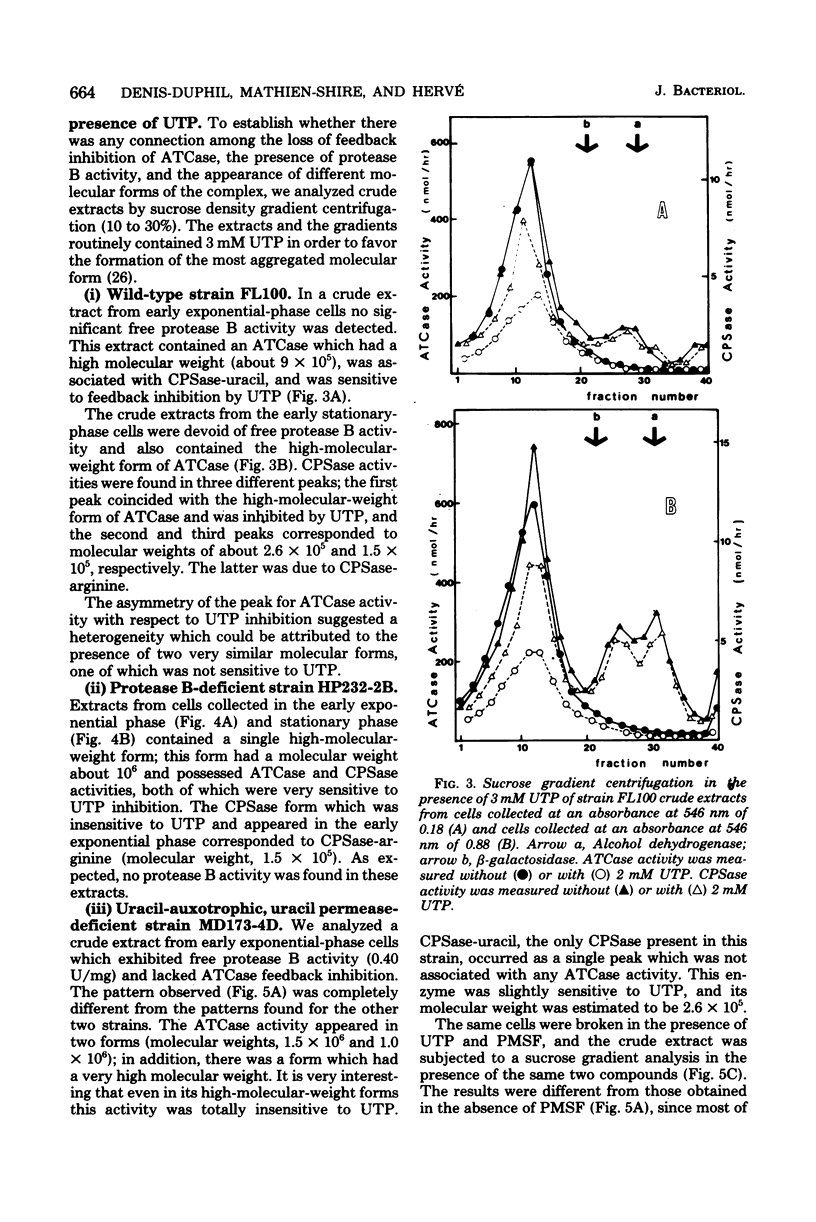
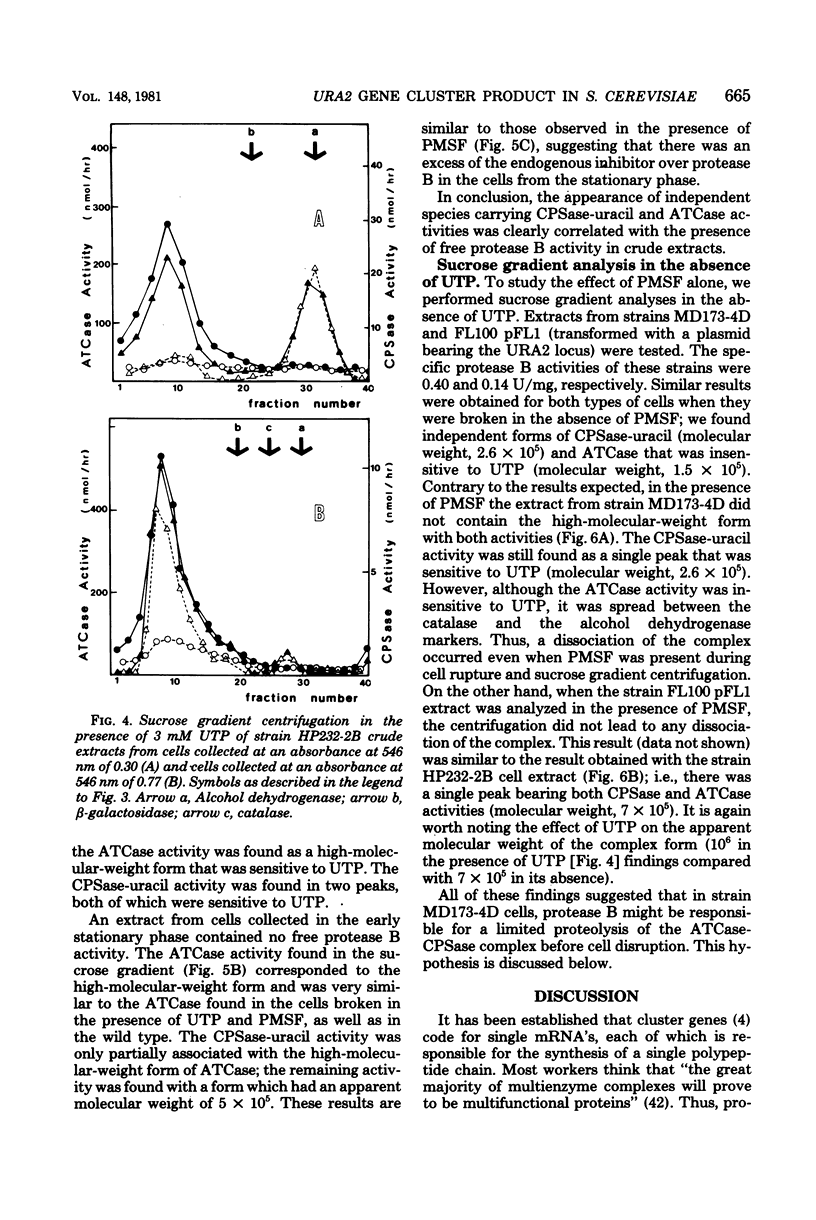
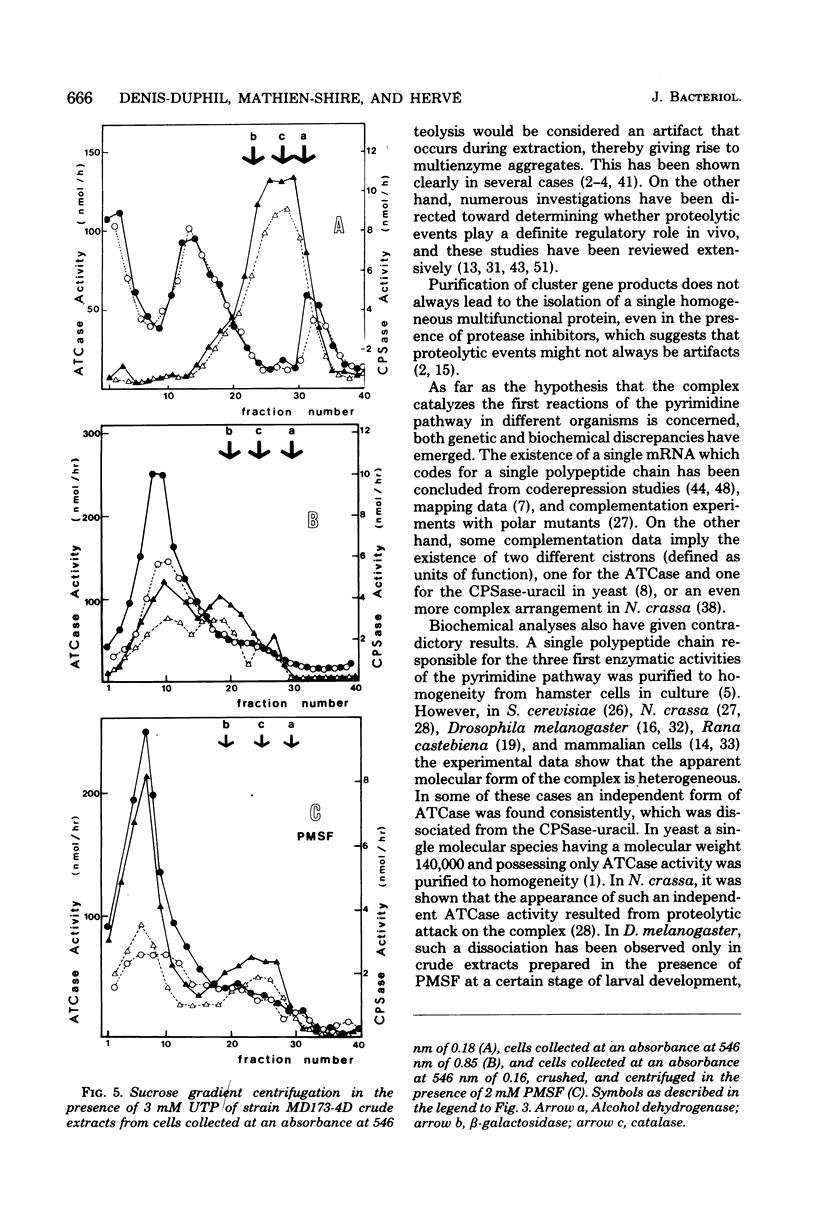
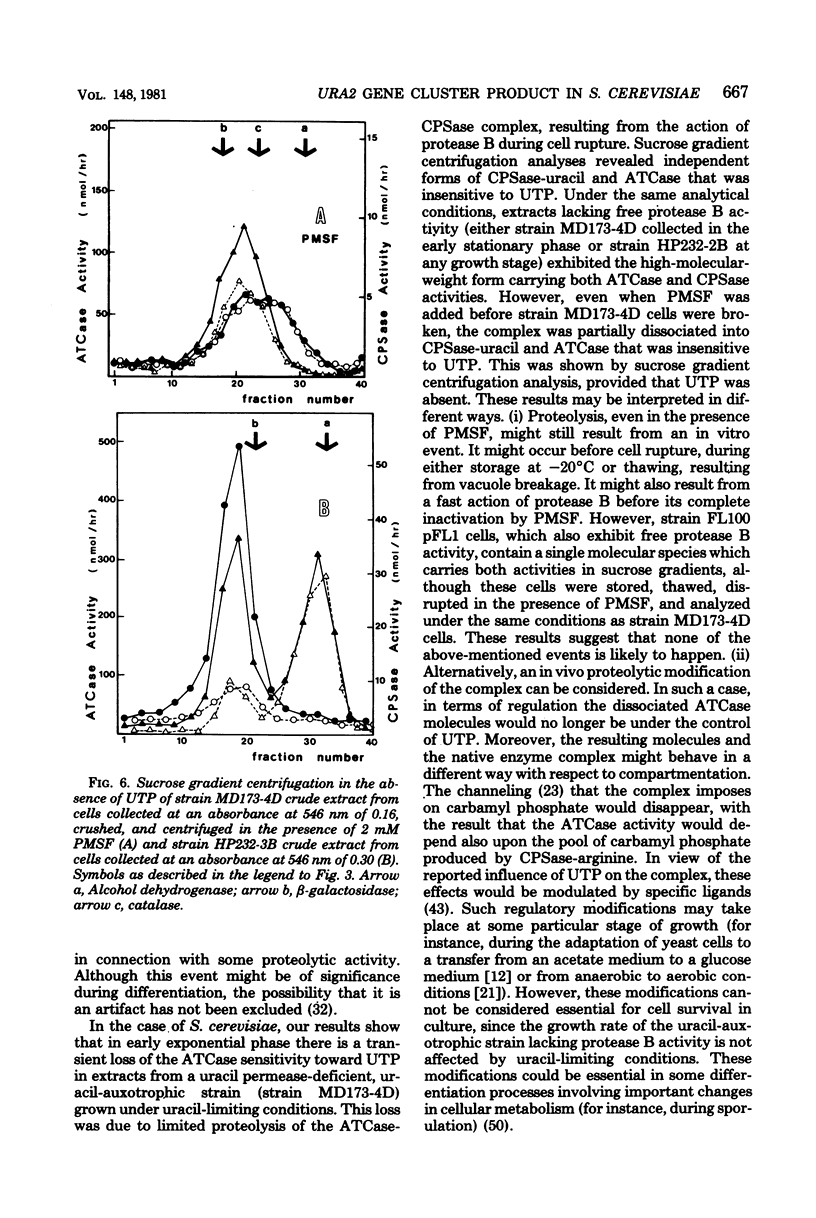
When a uracil-auxotrophic yeast strain is grown under uracil-limiting conditions, the aspartate transcarbamylase activity found in crude extracts shows a variation in sensitivity to feedback inhibition by uridine 5'-triphosphate. In this study we correlated this variation with changes in the molecular form of the carbamyl phosphate synthetase-uracil-aspartate transcarbamylase complex. Carbamyl phosphate synthetase-uracil (molecular weight, 240,000) and uridine 5'-triphosphate-insensitive aspartate transcarbamylase (molecular weight, 140,000) were present separately in extracts from cells collected in the early exponential phase; this was in contrast to the presence of a single high-molecular-weight form (molecular weight, about 900,000) bearing both activities in extracts from stationary-phase cells. The lack of sensitivity to uridine 5'-triphosphate by aspartate transcarbamylase was delayed by adding uridine 5'-triphosphate before cell disruption and was prevented completely by adding phenylmethylsulfonyl fluoride. Thus, this event was attributed to a transient serine protease activity detected only in early exponential-phase cell extracts. However, even in the presence of phenylmethylsulfonyl fluoride, a sucrose density gradient analysis in the absence of uridine 5'-triphosphate revealed a change in the aggregation state of the complex which might have occurred in vivo. None of these events was observed in extracts from cells that lacked protease B activity (strain HP232-2B).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken D. M., Bhatti A. R., Kaplan J. G. Characterization of the aspartate carbamoyltransferase subunit obtained from a multienzyme aggregate in the pyrimidine pathway of yeast. Activity and physical properties. Biochim Biophys Acta. 1973 May 5;309(1):50–57. doi: 10.1016/0005-2744(73)90316-1. [DOI] [PubMed] [Google Scholar]
- Bartholmes P., Böker H., Jaenicke R. Purification of tryptophan synthase from Saccharomyces cerevisiae and partial activity of its nicked subunits. Eur J Biochem. 1979 Dec;102(1):167–172. doi: 10.1111/j.1432-1033.1979.tb06277.x. [DOI] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Partial enzyme aggregates formed by pleiotropic mutants in the arom gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1971 Jan;68(1):58–62. doi: 10.1073/pnas.68.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Denis-Duphil M., Kaplan J. G. Fine structure of the URA2 locus in Saccharomyces cerevisiae. II. Meiotic and mitotic mapping studies. Mol Gen Genet. 1976 Jun 15;145(3):259–271. doi: 10.1007/BF00325822. [DOI] [PubMed] [Google Scholar]
- Denis-Duphil M., Lacroute F. Fine structure of the ura 2 locus in Saccharomyces cervisiae. I. In vivo complementation studies. Mol Gen Genet. 1971;112(4):354–364. doi: 10.1007/BF00334436. [DOI] [PubMed] [Google Scholar]
- Gaertner F. H., Cole K. W. A cluster-gene: evidence for one gene, one polypeptide, five enzymes. Biochem Biophys Res Commun. 1977 Mar 21;75(2):259–264. doi: 10.1016/0006-291x(77)91037-3. [DOI] [PubMed] [Google Scholar]
- Hansen R. J., Switzer R. L., Hinze H., Holzer H. Effects of glucose and nitrogen source on the levels of proteinases, peptidases, and proteinase inhibitors in yeast. Biochim Biophys Acta. 1977 Jan 24;496(1):103–114. doi: 10.1016/0304-4165(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Holzer H., Betz H., Ebner E. Intracellular proteinases in microorganisms. Curr Top Cell Regul. 1975;9:103–156. doi: 10.1016/b978-0-12-152809-6.50011-1. [DOI] [PubMed] [Google Scholar]
- Ishida H., Mori M., Tatibana M. Effects of dimethyl sulfoxide and glycerol on catalytic and regulatory properties of glutamine-dependent carbamoyl phosphate synthase from rat liver and dual effects of uridine triphosphate. Arch Biochem Biophys. 1977 Jul;182(1):258–265. doi: 10.1016/0003-9861(77)90306-x. [DOI] [PubMed] [Google Scholar]
- Jacobs P., Jauniaux J. C., Grenson M. A cis-dominant regulatory mutation linked to the argB-argC gene cluster in Saccharomyces cerevisiae. J Mol Biol. 1980 Jun 5;139(4):691–704. doi: 10.1016/0022-2836(80)90055-8. [DOI] [PubMed] [Google Scholar]
- Jarry B. P. Purification of aspartate transcarbamylase from Drosophila melanogaster. Eur J Biochem. 1978 Jul 3;87(3):533–540. doi: 10.1111/j.1432-1033.1978.tb12404.x. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G., Duphil M., Lacroute F. A study of the aspartate transcarbamylase activity of yeast. Arch Biochem Biophys. 1967 Mar;119(1):541–551. doi: 10.1016/0003-9861(67)90489-4. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G., Lacroute F., Messmer I. On the loss of feedback inhibition of yeast aspartate transcarbamylase during derepression of pyrimidine biosynthesis. Arch Biochem Biophys. 1969 Feb;129(2):539–544. doi: 10.1016/0003-9861(69)90212-4. [DOI] [PubMed] [Google Scholar]
- Kent R. J., Lin R. L., Sallach H. J., Cohen P. P. Reversible dissociation of a carbamoyl phosphate synthase-aspartate transcarbamoylase-dihydroorotase complex from ovarian eggs of Rana catesbeiana: effect of uridine triphosphate and other modifiers. Proc Natl Acad Sci U S A. 1975 May;72(5):1712–1716. doi: 10.1073/pnas.72.5.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner K., Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- Klein H. P., Jahnke L. Effects of aeration on formation and localization of the acetyl coenzyme A synthetases of Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):179–184. doi: 10.1128/jb.137.1.179-184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Aggregation states of a regulatory enzyme complex catalyzing the early steps of pyrimidine biosynthesis in bakers' yeast. Can J Biochem. 1971 Apr;49(4):403–411. doi: 10.1139/o71-059. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Heat-induced disaggregation of a multifunctional enzyme complex catalyzing the first steps in pyrimidine biosynthesis in bakers' yeast. Can J Biochem. 1970 Feb;48(2):155–159. doi: 10.1139/o70-024. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Metabolic compartmentation at the molecular level: the function of a multienzyme aggregate in the pyrimidine pathway of yeast. Biochim Biophys Acta. 1970 Dec 16;220(3):365–372. doi: 10.1016/0005-2744(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. The aspartate transcarbamylase and carbamoyl phosphate synthetase of yeast: a multi-functional enzyme complex. Biochem Biophys Res Commun. 1969 Feb 21;34(4):426–433. doi: 10.1016/0006-291x(69)90399-4. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Buxton F. P., Radford A. A possible model for the structure of the Neurospora carbamoyl phosphate synthase-aspartate carbamoyl transferase complex enzyme. Mol Gen Genet. 1978 May 31;161(3):297–304. doi: 10.1007/BF00331004. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Radford A. Dissociation of an enzyme complex from Neurospora crassa. Evidence for a protease. Biochim Biophys Acta. 1977 Dec 8;485(2):314–329. doi: 10.1016/0005-2744(77)90167-x. [DOI] [PubMed] [Google Scholar]
- Matchett W. H., DeMoss J. A. The subunit structure of tryptophan synthase from Neurospora crassa. J Biol Chem. 1975 Apr 25;250(8):2941–2946. [PubMed] [Google Scholar]
- Matchett W. H. Indole channeling by tryptophan synthase of neurospora. J Biol Chem. 1974 Jul 10;249(13):4041–4049. [PubMed] [Google Scholar]
- Maurizi M. R., Switzer R. L. Proteolysis in bacterial sporulation. Curr Top Cell Regul. 1980;16:163–224. doi: 10.1016/b978-0-12-152816-4.50010-8. [DOI] [PubMed] [Google Scholar]
- Mehl Y., Jarry B. P. Developmental regulation of the first three enzymes of pyrimidine biosynthesis in Drosophila melanogaster. Dev Biol. 1978 Nov;67(1):1–10. doi: 10.1016/0012-1606(78)90295-6. [DOI] [PubMed] [Google Scholar]
- Mori M., Tatibana M. Multi-enzyme complex of glutamine-dependent carbamoyl-phosphate synthetase with aspartate carbamoyltransferase and dihydroorotase from rat ascites-hepatoma cells. Purification, molecular properties and limited proteolysis. Eur J Biochem. 1978 May 16;86(2):381–388. doi: 10.1111/j.1432-1033.1978.tb12320.x. [DOI] [PubMed] [Google Scholar]
- Nester E. W., Lorence J. H., Nasser D. S. An enzyme aggregate involved in the biosynthesis of aromatic amino acids in Bacillus subtilis. Its possible function in feedback regulation. Biochemistry. 1967 May;6(5):1553–1563. doi: 10.1021/bi00857a042. [DOI] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Pringle J. R. Methods for avoiding proteolytic artefacts in studies of enzymes and other proteins from yeasts. Methods Cell Biol. 1975;12:149–184. doi: 10.1016/s0091-679x(08)60956-5. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L. Spectrum of forward mutants in the pyr-3 region of Neurospora. J Gen Microbiol. 1963 Feb;30:327–337. doi: 10.1099/00221287-30-2-327. [DOI] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Comparisons of the tryptophan synthase inactivating enzymes with proteinases from yeast. Eur J Biochem. 1974 Mar 1;42(2):621–626. doi: 10.1111/j.1432-1033.1974.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Proteolytic activities in yeast. Biochim Biophys Acta. 1975 Mar 28;384(1):203–214. doi: 10.1016/0005-2744(75)90109-6. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Welch G. R., Gaertner F. H. Enzyme organization in the polyaromatic-biosynthetic pathway: the arom conjugate and other multienzyme systems. Curr Top Cell Regul. 1980;16:113–162. [PubMed] [Google Scholar]
- Welch G. R., Gaertner F. H. Influence of an aggregated multienzyme system on transient time: kinetic evidence for compartmentation by an aromatic-amino-acid synthesizing complex of Neurospora crassa. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4218–4222. doi: 10.1073/pnas.72.11.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S., Davis R. H. Copurification of pyrimidine-specific carbamyl phosphate synthetase and aspartate transcarbamylase of Neurospora crassa. Biochemistry. 1970 Oct 27;9(22):4329–4335. doi: 10.1021/bi00824a013. [DOI] [PubMed] [Google Scholar]
- Wolf D. H. Control of metabolism in yeast and other lower eukaryotes through action of proteinases. Adv Microb Physiol. 1980;21:267–338. doi: 10.1016/s0065-2911(08)60358-6. [DOI] [PubMed] [Google Scholar]
- Wolf D. H., Ehmann C. Studies on a proteinase B mutant of yeast. Eur J Biochem. 1979 Aug 1;98(2):375–384. doi: 10.1111/j.1432-1033.1979.tb13197.x. [DOI] [PubMed] [Google Scholar]


