Abstract
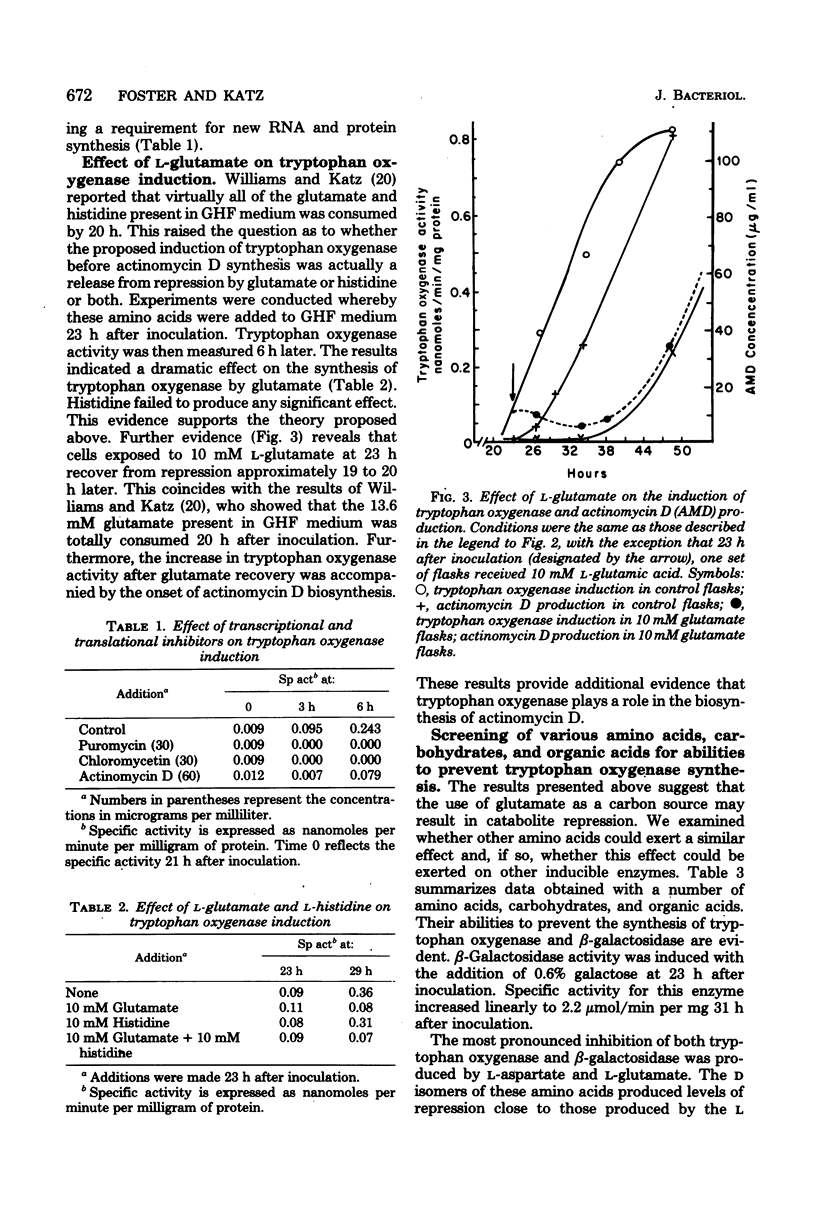
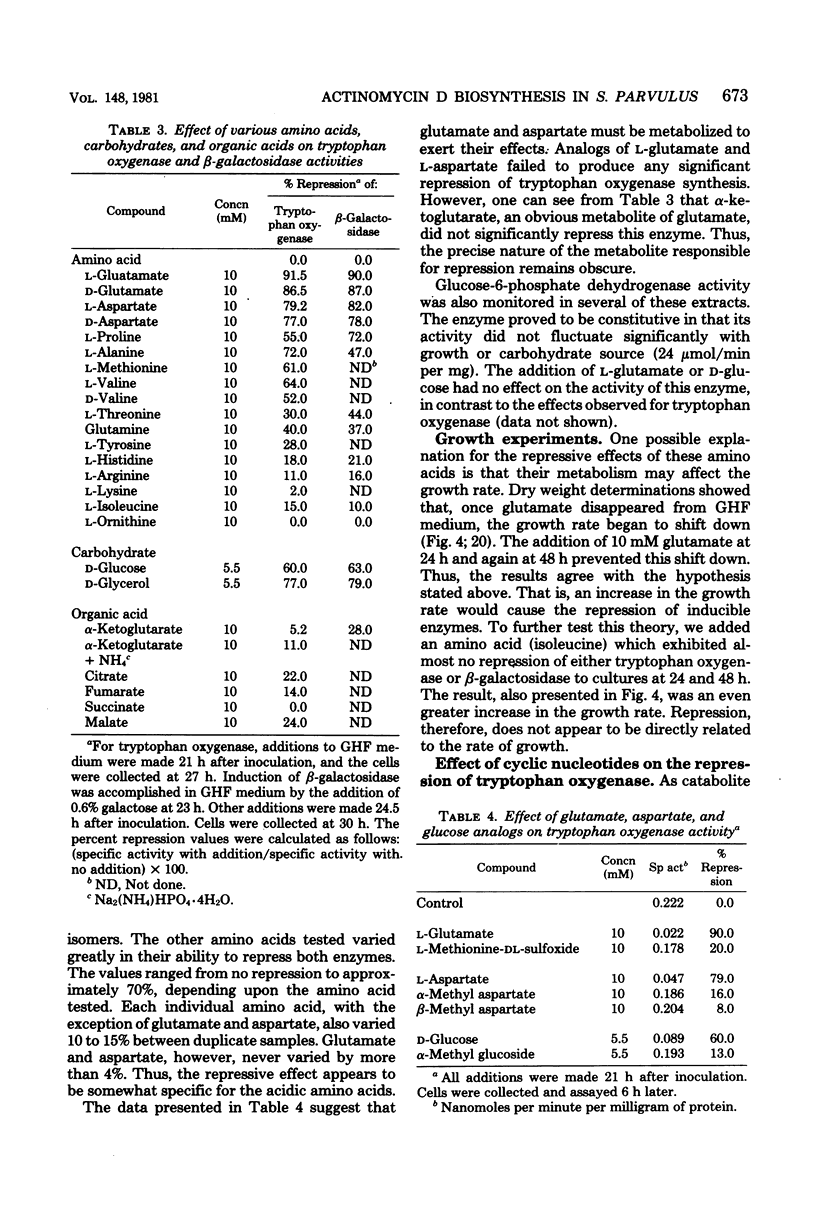
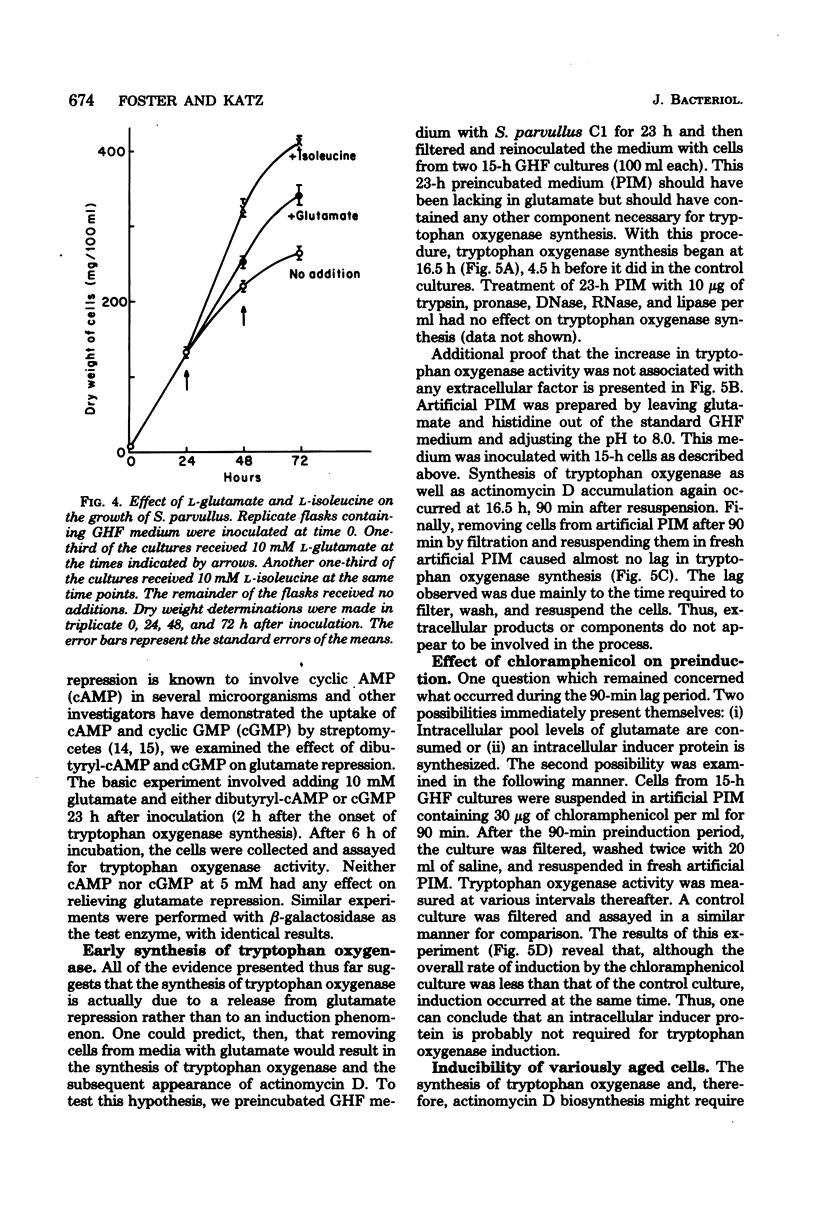
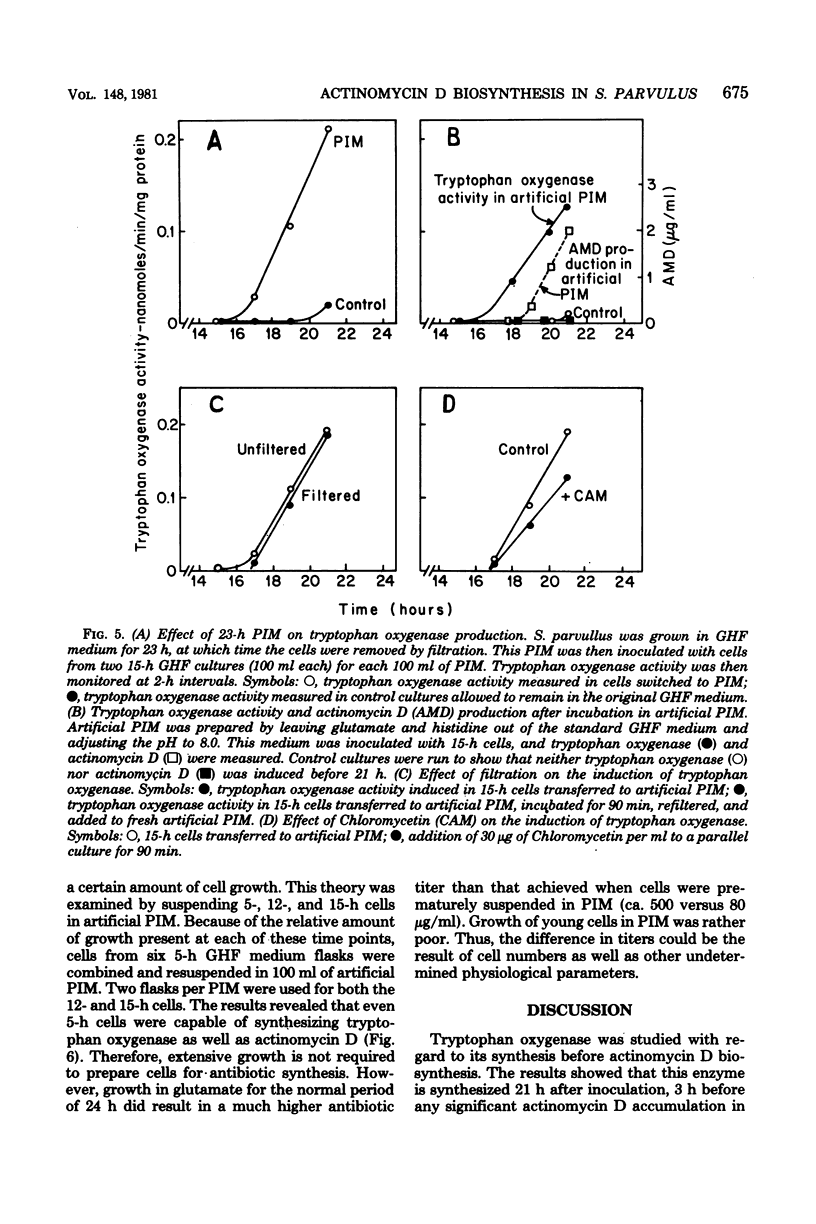
Tryptophan oxygenase (tryptophan 2,3-dioxygenase) activity increases immediately before the initiation of actinomycin D production by Streptomyces parvullus. We have attempted to discern whether this increase is due to a release from catabolite repression or to the synthesis of an inducer substance. The standard culture medium (glutamic acid-histidine-fructose medium) used in antibiotic production studies with S. parvullus contains l-glutamate as a major constituent. l-Glutamate is almost totally consumed before the onset of actinomycin D synthesis. The addition of 10 mM l-glutamate at this stage completely abolished actinomycin D production as well as tryptophan oxygenase synthesis. Fourteen amino acids were tested for a similar effect. Of these, l-glutamate and l-aspartate had the most dramatic effect on tryptophan oxygenase and β-galactosidase (β-d-galactosidase), another inducible enzyme. Standard glutamic acid-histidine-fructose medium, preincubated for 23 h to remove l-glutamate, allowed the synthesis of actinomycin D and tryptophan oxygenase by cells at a stage of growth normally considered too early for antibiotic production. A chemically defined medium lacking l-glutamate and adjusted to pH 8.0 was designed to simulate the preincubation medium. The transfer of cells to this artificial preincubation medium resulted in the appearance of tryptophan oxygenase as early as 19 h before normal synthesis occurred, eliminating the possibility that an inducer molecule is synthesized and excreted during the preincubation period. The results of these studies suggest that the increase in tryptophan oxygenase activity before the onset of actinomycin D synthesis, as well as the synthesis of actinomycin D itself, is due to a release from l-glutamate catabolite repression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Bouknight R. R., Sadoff H. L. Tryptophan catabolism in Bacillus megaterium. J Bacteriol. 1975 Jan;121(1):70–76. doi: 10.1128/jb.121.1.70-76.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Hitchcock M. J., Katz E. Evidence for a constitutive and inducible form of kynurenine formamidase in an actinomycin-producing strain of Streptomyces parvulus. Arch Biochem Biophys. 1980 Jun;202(1):18–22. doi: 10.1016/0003-9861(80)90400-2. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Evidence against the presence of cyclic AMP and related enzymes in selected strains of Bacteroides fragilis. Biochem Biophys Res Commun. 1974 Sep 9;60(1):88–95. doi: 10.1016/0006-291x(74)90176-4. [DOI] [PubMed] [Google Scholar]
- KATZ E., WEISSBACH H. Incorporation of C14-labeled amino acids into actinomycin and protein by Streptomyces antibioticus. J Biol Chem. 1963 Feb;238:666–675. [PubMed] [Google Scholar]
- KATZ E., WISE M., WEISSBACH H. ACTINOMYCIN BIOSYNTHESIS. DIFFERENTIAL EFFECT OF CHLORAMPHENICOL ON PROTEIN AND PEPTIDE ANTIBIOTIC SYNTHESIS. J Biol Chem. 1965 Jul;240:3071–3078. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lingens F., Vollprecht P. Zur Biosynthese der Nicotinsäure in Streptomyceten, Algen, Phycomyceten und Hefe. Hoppe Seylers Z Physiol Chem. 1964;339(1):64–74. [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Meienhofer J., Atherton E. Structure-activity relationships in the actinomycins. Adv Appl Microbiol. 1973;16:203–300. [PubMed] [Google Scholar]
- Peterkofsky B. Use of a new radioassay for tryptophan oxygenase to study the development of the enzyme in chick embryos. Arch Biochem Biophys. 1968 Dec;128(3):637–645. doi: 10.1016/0003-9861(68)90073-8. [DOI] [PubMed] [Google Scholar]
- Ring K., Langheinrich W., Ehle H., Foit B. Effect of dibutyryl cyclic adenosine monophosphate on active amino acid transport in Streptomyces hydrogenans. Arch Microbiol. 1977 Nov 18;115(2):199–205. doi: 10.1007/BF00406375. [DOI] [PubMed] [Google Scholar]
- Rosenfeld H., Feigelson P. Synergistic and product induction of the enzymes of tryptophan metabolism in Pseudomonas acidovorans. J Bacteriol. 1969 Feb;97(2):697–704. doi: 10.1128/jb.97.2.697-704.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Inability of detect cyclic AMP in vegetative or sporulating cells or dormant spores of Bacillus megaterium. Biochem Biophys Res Commun. 1973 May 15;52(2):365–372. doi: 10.1016/0006-291x(73)90720-1. [DOI] [PubMed] [Google Scholar]
- Siegel L. S., Hylemon P. B., Phibbs P. V., Jr Cyclic adenosine 3',5'-monophosphate levels and activities of adenylate cyclase and cyclic adenosine 3',5'-monophosphate phosphodiesterase in Pseudomonas and Bacteroides. J Bacteriol. 1977 Jan;129(1):87–96. doi: 10.1128/jb.129.1.87-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troost T., Katz E. Phenoxazinone biosynthesis: accumulation of a precursor, 4-methyl-3-hydroxyanthranilic acid, by mutants of Streptomyces parvulus. J Gen Microbiol. 1979 Mar;111(1):121–132. doi: 10.1099/00221287-111-1-121. [DOI] [PubMed] [Google Scholar]
- Williams W. K., Katz E. Development of a chemically defined medium for the synthesis of actinomycin D by Streptomyces parvulus. Antimicrob Agents Chemother. 1977 Feb;11(2):281–290. doi: 10.1128/aac.11.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung K. H., Larsson G. C., Yamazaki H. Evidence against the involvement of adenosine 3',5'-cyclic monophosphate in glucose inhibition of beta-galactosidase induction in Bacillus megaterium. Can J Biochem. 1976 Oct;54(10):854–865. doi: 10.1139/o76-123. [DOI] [PubMed] [Google Scholar]


