Abstract
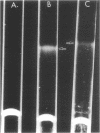
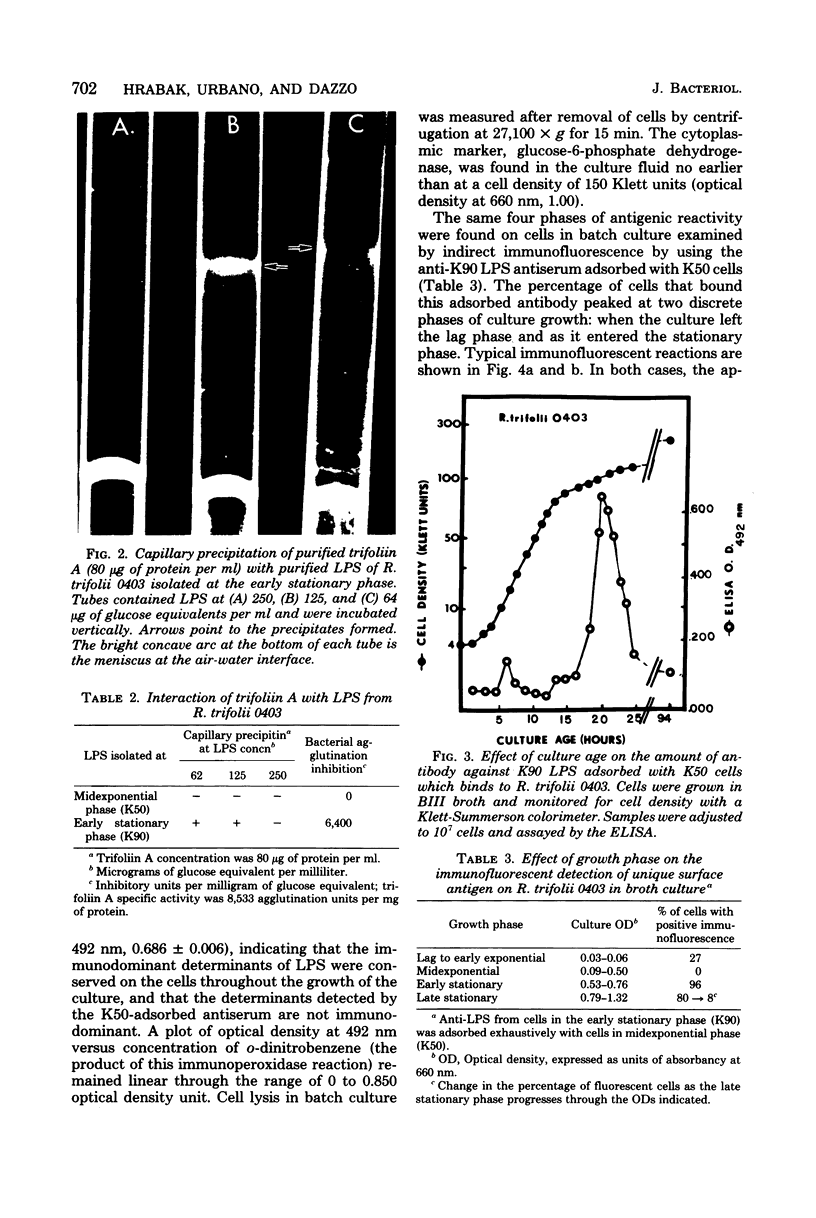
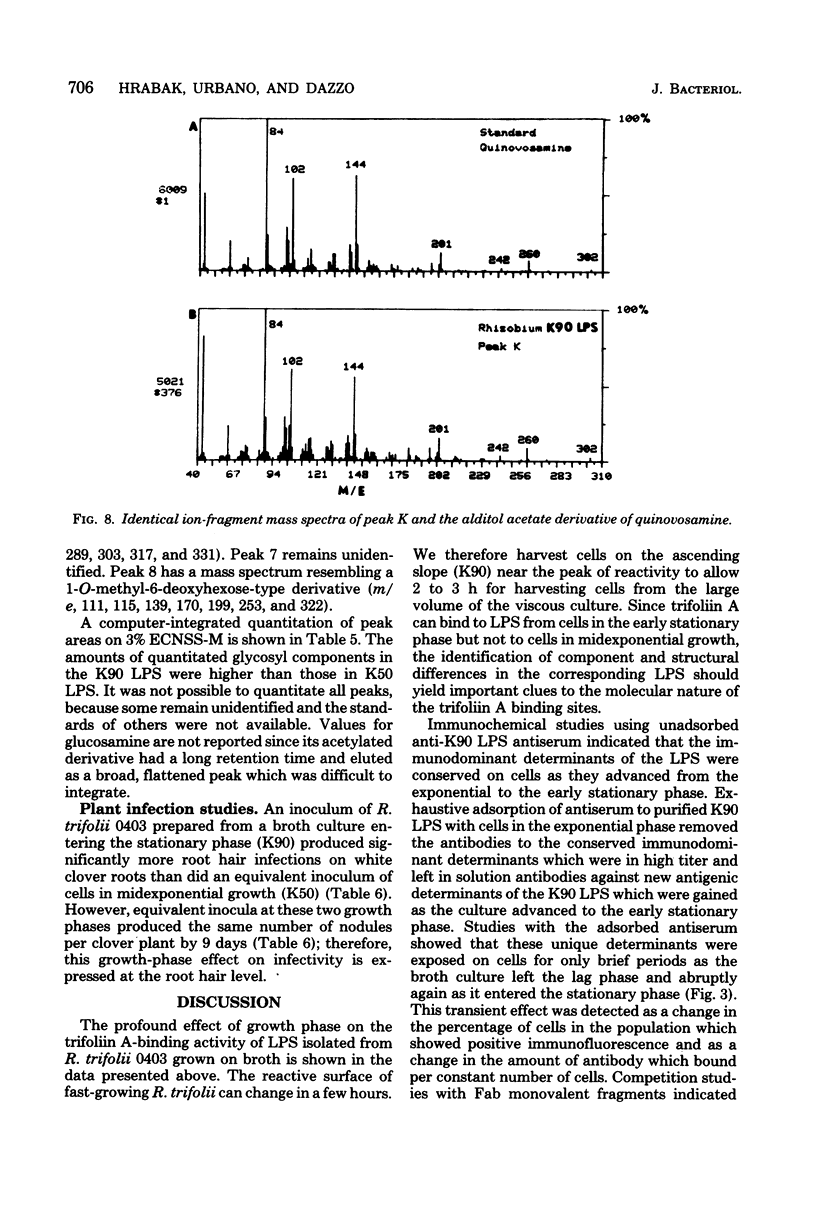
The lipopolysaccharide (LPS) from Rhizobium trifolii 0403 was isolated at different stages of growth and was examined for its (i) ability to bind a white clover lectin (trifoliin A), (ii) immunochemical properties, and (iii) composition. There was significantly more binding of trifoliin A to purified LPS and cells in the early stationary phase than to cells in the exponential phase. Immunofluorescence and enzyme-linked immunosorbent assays indicated that new antigenic determinants of the LPS appeared for brief periods on cells at the end of the lag phase and again at the beginning of the stationary phase. These new antigens were not detected on cells in midexponential or late stationary phase. Monovalent fragments of immunoglobulin G antibodies raised against the unique antigenic determinants in the LPS competitively blocked the binding of trifoliin A to cells in the early stationary phase. Gas chromatographic analysis showed that the relative quantity of several glycosyl components in the LPS increased as the culture advanced from the midexponential to the early stationary phase. In addition, LPS from cells in the early stationary phase had a higher aggregate molecular weight. Quinovosamine (2-amino-2,6-dideoxyglucose) was identified by combined gas chromatography-mass spectrometry as a sugar component of the LPS which had not been previously reported. D-Quinovosamine, N-acetyl-D-quinovosamine, and its n-propyl-beta-glycoside were effective hapten sugars which inhibited the binding of trifoliin A, anti-clover root antibody, and homologous antibody to these new determinants in the LPS. White clover plants had more infected root hairs after incubation with an inoculum of cells in the early stationary phase than after incubation with cells in the midexponential phase. The profound influence of the growth phase on the composition of lectin-binding polysaccharides of Rhizobium may be a major underlying cause of conflicting data among laboratories testing the lectin-recognition hypothesis. In addition, these chemical modifications may reflect mechanisms which regulate Rhizobium-root hair recognition in this nitrogen-fixing symbiosis.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Ayers A. R., Valent B. S., Ebel J., Hahn M., Wolpert J., Carlson R. Plants interact with microbial polysaccharides. J Supramol Struct. 1977;6(4):599–616. doi: 10.1002/jss.400060413. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: III. Influence of Rhizosphere/Rhizoplane Culture Conditions on the Soybean Lectin-binding Properties of Rhizobia. Plant Physiol. 1978 Jul;62(1):71–74. doi: 10.1104/pp.62.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Pueppke S. G., Bauer W. D. Role of lectins in plant-microorganism interactions: I. Binding of soybean lectin to rhizobia. Plant Physiol. 1977 Oct;60(4):486–491. doi: 10.1104/pp.60.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Deinema M. H., Zevenhuizen L. P. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol. 1971;78(1):42–51. doi: 10.1007/BF00409087. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planqué K., Kijne J. W. Binding of pea lectins to a glycan type polysaccharide in the cell walls of Rhizobium leguminosarum. FEBS Lett. 1977 Jan 15;73(1):64–66. doi: 10.1016/0014-5793(77)80016-1. [DOI] [PubMed] [Google Scholar]
- Schlecht S., Fromme I. Chemische und serologische Eigenschaften von Salmonella-Lipopolysacchariden aus unterschiedlichen Wachstumsphasen. Zentralbl Bakteriol Orig A. 1975 Oct;233(2):199–222. [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Bodwin J. S., Lippincott B. B., Lippincott J. A. Role of Agrobacterium cell envelope lipopolysaccharide in infection site attachment. Infect Immun. 1976 Apr;13(4):1080–1083. doi: 10.1128/iai.13.4.1080-1083.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]
- Zevenhuizen L. P., Scholten-Koerselman H. J. Surface carbohydrates of Rhizobium. I. Beta-1, 2-glucans. Antonie Van Leeuwenhoek. 1979;45(2):165–175. doi: 10.1007/BF00418581. [DOI] [PubMed] [Google Scholar]
- Zurkowski W. Specific adsorption of bacteria to clover root hairs, related to the presence of the plasmid pWZ2 in cells of Rhizobium trifolii. Microbios. 1980;27(107):27–32. [PubMed] [Google Scholar]