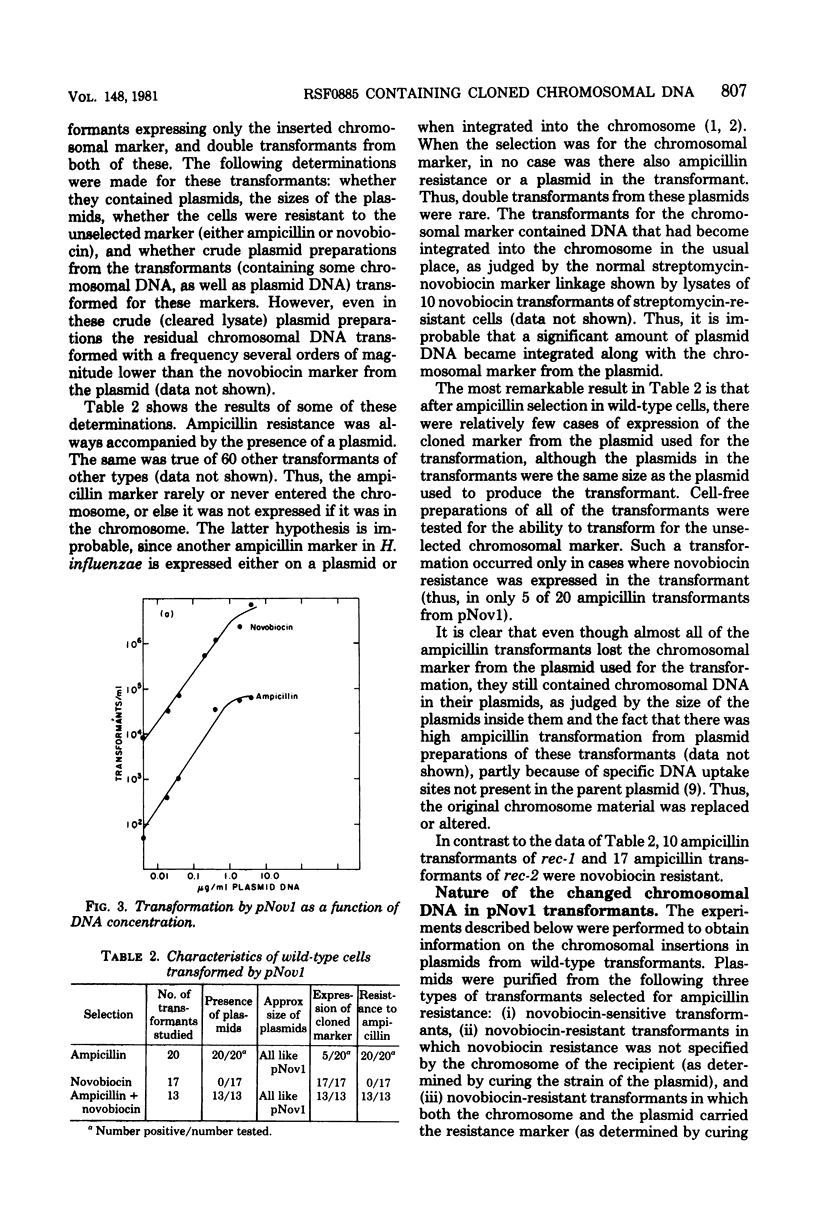
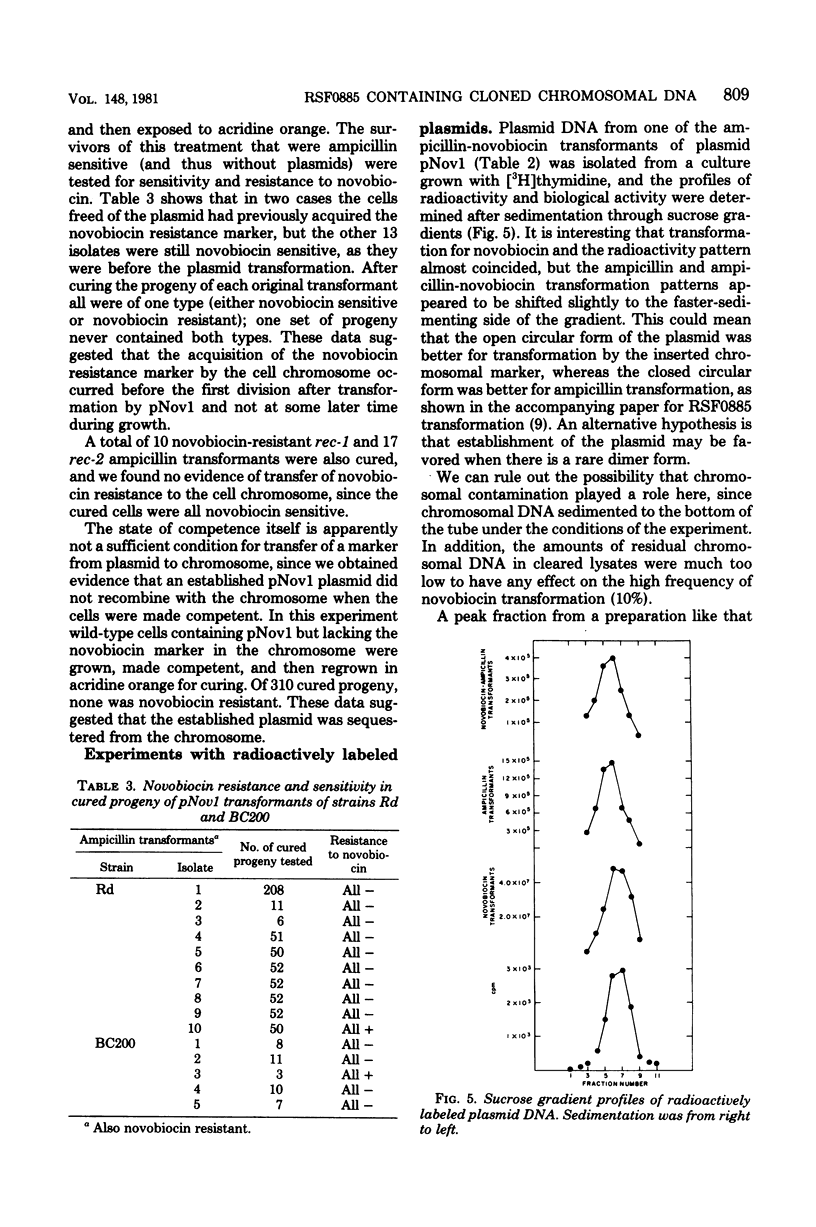
Abstract
A plasmid containing a single cloned insertion of Haemophilus influenzae chromosomal deoxyribonucleic acid that carried a novobiocin resistance marker was 2.6 times larger than the parent plasmid, RSF0885, which conferred ampicillin resistance. The most frequent type of transformation by this plasmid (designated pNov1) was the transfer of novobiocin resistance to the chromosome, with the loss of the plasmid from the recipient. In accord with this observation, after radioactively labeled pNov1 entered a competent cell, it lost acid-insoluble counts, as well as biological activity. The level of ampicillin transformation, which involved establishment of the plasmid, was almost two orders of magnitude lower than the level of novobiocin transformation. Both types of transformation were depressed profoundly in rec-1 and rec-2 mutants. Ampicillin transformants of wild-type cells always contained plasmids that were the same size as pNov1, although most of these transformants were not novobiocin resistant. Plasmid pNov1 in wild-type cells but not in rec-1 or rec-2 cells often recombined with the chromosome, causing a homologous region of the chromosome to be substituted for part of the plasmid, as shown by restriction and genetic analyses. Our data suggested that plasmid-chromosome recombination took place only around the time when the plasmid entered a cell, rather than after it became established.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Bendler J. W., Setlow J. K. Plasmid transformation in Haemophilus influenzae. J Bacteriol. 1981 Feb;145(2):1099–1101. doi: 10.1128/jb.145.2.1099-1101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendler J. W., 3rd Physical size of the donor locus and transmission of Haemophilus influenzae ampicillin resistance genes by deoxyribonucleic acid-mediated transformation. J Bacteriol. 1976 Jan;125(1):197–204. doi: 10.1128/jb.125.1.197-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- De Graaff J., Elwell L. P., Falkow S. Molecular nature of two beta-lactamase-specifying plasmids isolated from Haemophilus influenzae type b. J Bacteriol. 1976 Apr;126(1):439–446. doi: 10.1128/jb.126.1.439-446.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Setlow J. K. Single-strand regions in the deoxyribonucleic acid of competent Haemophilus influenzae. J Bacteriol. 1975 Jun;122(3):1091–1102. doi: 10.1128/jb.122.3.1091-1102.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885. J Bacteriol. 1981 Dec;148(3):812–816. doi: 10.1128/jb.148.3.812-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Elwell L. P., Falkow S. Molecular characterization of two beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae. J Bacteriol. 1977 Aug;131(2):557–563. doi: 10.1128/jb.131.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]