Abstract
Transcription is known to be coupled to translation in many or all bacterial operons which code for proteins. In these operons, nonsense codons which prevent normal translation often result in premature termination of transcription (polarity). However, efficient transcription of ribosomal ribonucleic acid operons (rrn operons) occurs, although rrn transcripts are not translated. It therefore seemed possible that insertion sequences and transposable elements which are polar in protein-coding operons might not be polar in rrn operons. Previously, it has been shown (E. A. Morgan, Cell 21:257-265, 1980) that Tn10 is incompletely polar in the rrnX operon. Here we show that the transposon Tn9 and the insertion sequence IS1 also incompletely polar in rrnX. In normal cells expression of sequences distal to the insertions can be detected by genetic methods. In ultraviolet-irradiated cells expression of distal sequences is about 80% of that observed in uninterrupted rrnX operons. These observations provide evidence that ribonucleic acid polymerase molecules beginning at rrnX promoters can read through Tn9 and IS1 and that, at least in ultraviolet-irradiated cells, read-through is very efficient.
Full text
PDF

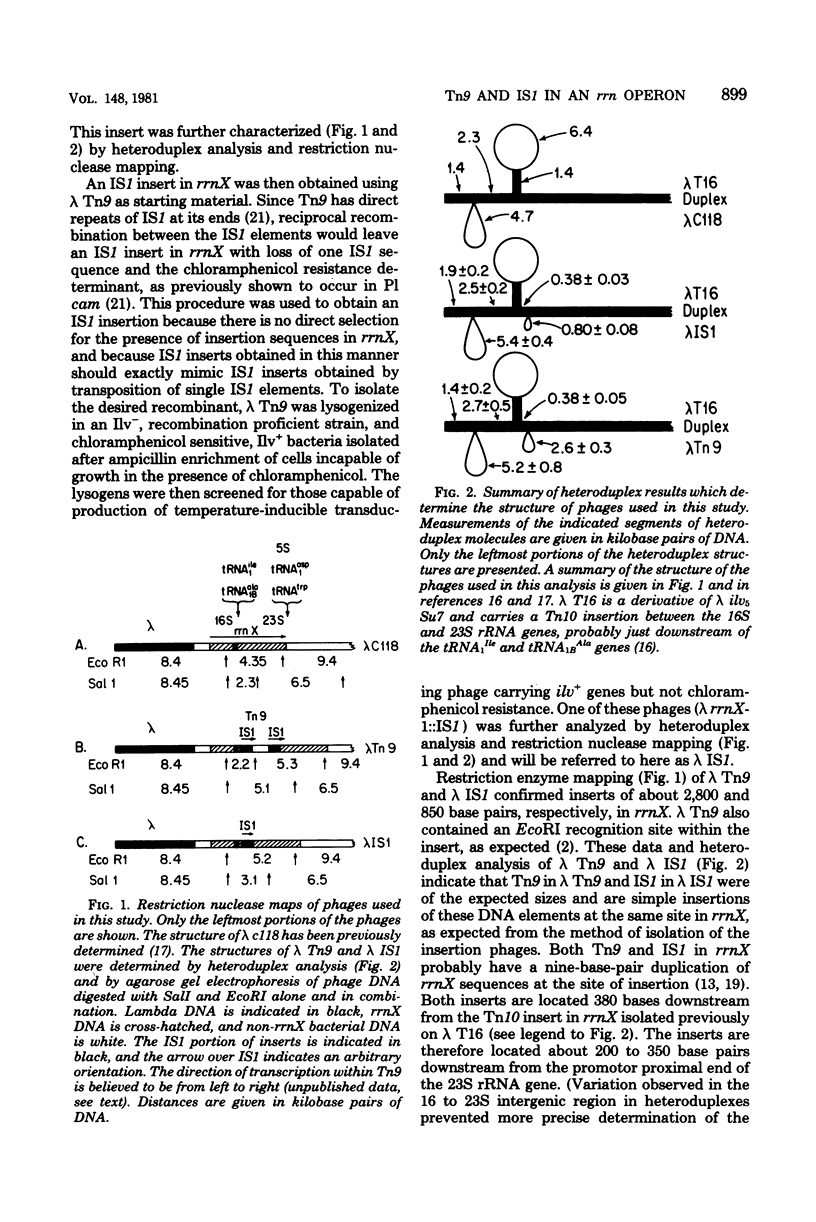
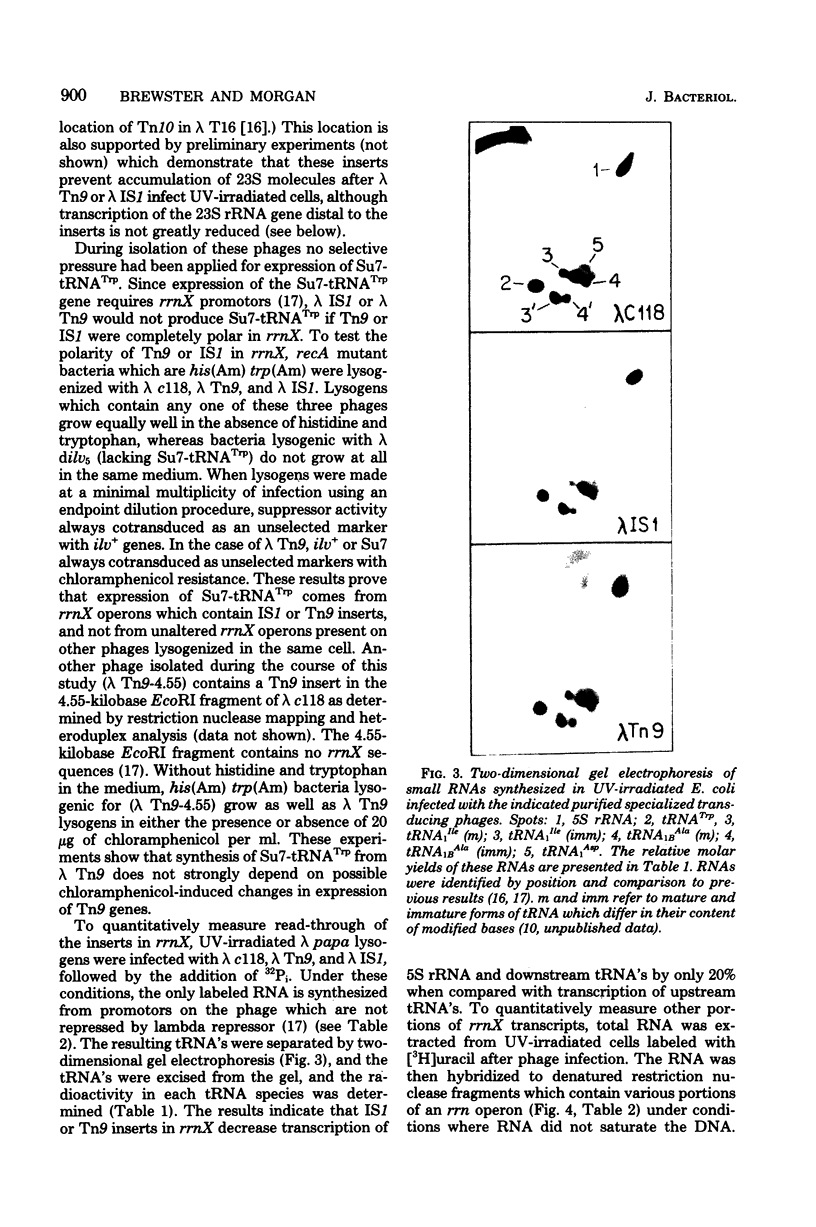
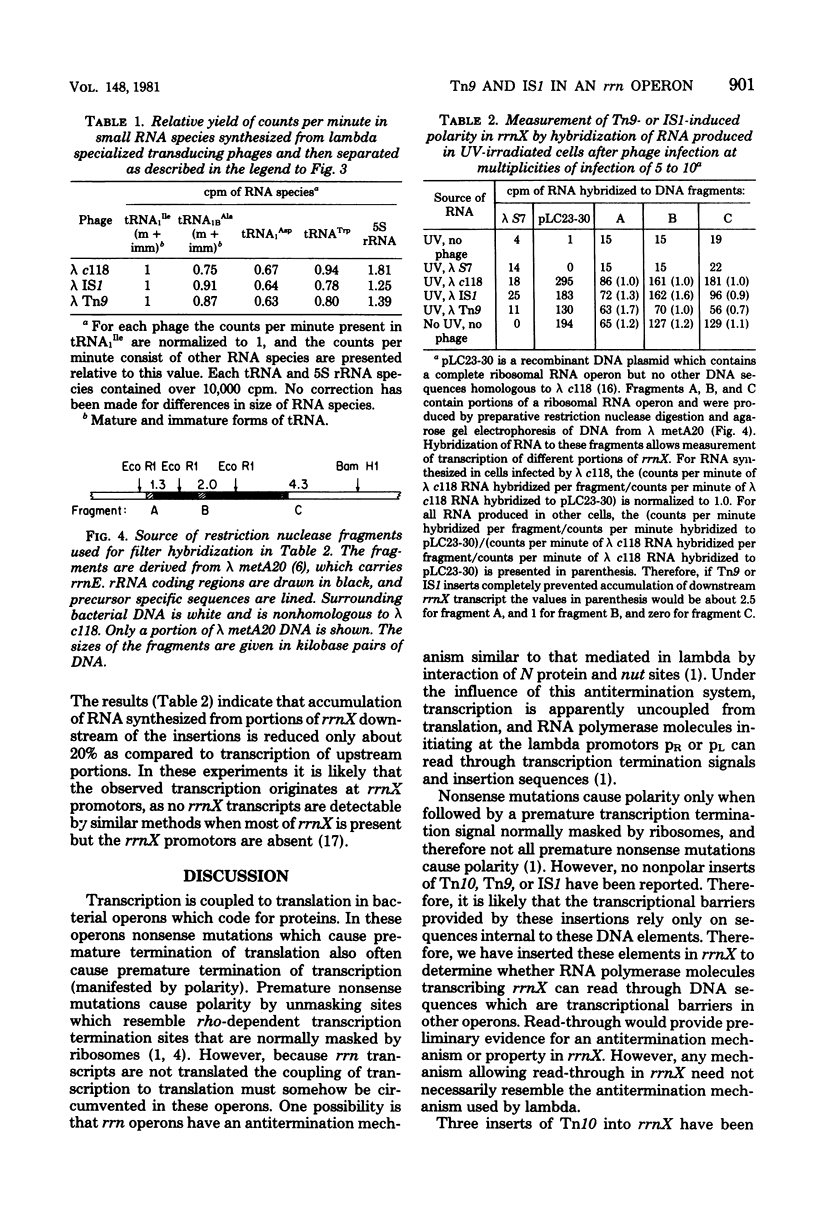


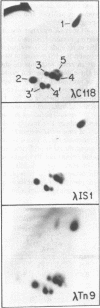
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Cabezón T., Delcuve G., Faelen M., Desmet L., Bollen A. Polarity of amber mutations in ribosomal protein genes of Escherichia coli. J Bacteriol. 1980 Jan;141(1):41–51. doi: 10.1128/jb.141.1.41-51.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calva E., Burgess R. R. Characterization of a rho-dependent termination site within the cro gene of bacteriophage lambda. J Biol Chem. 1980 Nov 25;255(22):11017–11022. [PubMed] [Google Scholar]
- FRANKLIN N. C., LURIA S. E. Transduction by bacteriophage P-1 and the properties of the lac genetic region in E. coli and S. dysenteriae. Virology. 1961 Nov;15:299–311. doi: 10.1016/0042-6822(61)90362-2. [DOI] [PubMed] [Google Scholar]
- Fassler J. S., Tessman I. Suppression of polarity in the gal operon by ultraviolet irradiation. J Bacteriol. 1980 Dec;144(3):1205–1207. doi: 10.1128/jb.144.3.1205-1207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandt M., Szybalski W., Malamy M. H. Polar mutations in lac, gal and phage lambda consist of a few IS-DNA sequences inserted with either orientation. Mol Gen Genet. 1972;119(3):223–231. doi: 10.1007/BF00333860. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Nomura M. Expression of spacer tRNA genes in ribosomal RNA transcription units carried by hybrid Col E1 plasmids in E. coli. Cell. 1977 Aug;11(4):779–793. doi: 10.1016/0092-8674(77)90291-4. [DOI] [PubMed] [Google Scholar]
- Johnsrud L., Calos M. P., Miller J. H. The transposon Tn9 generates a 9 bp repeated sequence during integration. Cell. 1978 Dec;15(4):1209–1219. doi: 10.1016/0092-8674(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. A. Insertions of Tn 10 into an E. coli ribosomal RNA operon are incompletely polar. Cell. 1980 Aug;21(1):257–265. doi: 10.1016/0092-8674(80)90133-6. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Nomura M. Deletion analysis of the expression of rRNA genes and associated tRNA genes carried by a lambda transducing bacteriophage. J Bacteriol. 1979 Jan;137(1):507–516. doi: 10.1128/jb.137.1.507-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Morgan E. A. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- Ohtsubo H., Ohtsubo E. Nucleotide sequence of an insertion element, IS1. Proc Natl Acad Sci U S A. 1978 Feb;75(2):615–619. doi: 10.1073/pnas.75.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock T. J., Tessman E. S., Tessman I. Suppression of polar effects of nonsense mutations by ultraviolet irradiation. J Bacteriol. 1979 Apr;138(1):122–125. doi: 10.1128/jb.138.1.122-125.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Gilbert S. F., Nomura M. DNA sequences of promoter regions for rRNA operons rrnE and rrnA in E. coli. Cell. 1979 May;17(1):201–209. doi: 10.1016/0092-8674(79)90308-8. [DOI] [PubMed] [Google Scholar]