Abstract
Coenzyme A (CoA) and acyl carrier protein are two cofactors in fatty acid metabolism, and both possess a 4'-phosphopantetheine moiety that is metabolically derived from the vitamin pantothenate. We studied the regulation of the metabolic pathway that gives rise to these two cofactors in an Escherichia coli beta-alanine auxotroph, strain SJ16. Identification and quantitation of the intracellular and extracellular beta-alanine-derived metabolites from cells grown on increasing beta-alanine concentrations were performed. The intracellular content of acyl carrier protein was relatively insensitive to beta-alanine input, whereas the CoA content increased as a function of external beta-alanine concentration, reaching a maximum at 8 microM beta-alanine. Further increase in the beta-alanine concentration led to the excretion of pantothenate into the medium. Comparing the amount of pantothenate found outside the cell to the level of intracellular metabolites demonstrates that E. coli is capable of producing 15-fold more pantoic acid than is required to maintain the intracellular CoA content. Therefore, the supply of pantoic acid is not a limiting factor in CoA biosynthesis. Wild-type cells also excreted pantothenate into the medium, showing that the beta-alanine supply is also not rate limiting in CoA biogenesis. Taken together, the results point to pantothenate kinase as the primary enzymatic step that regulates the CoA content of E. coli.
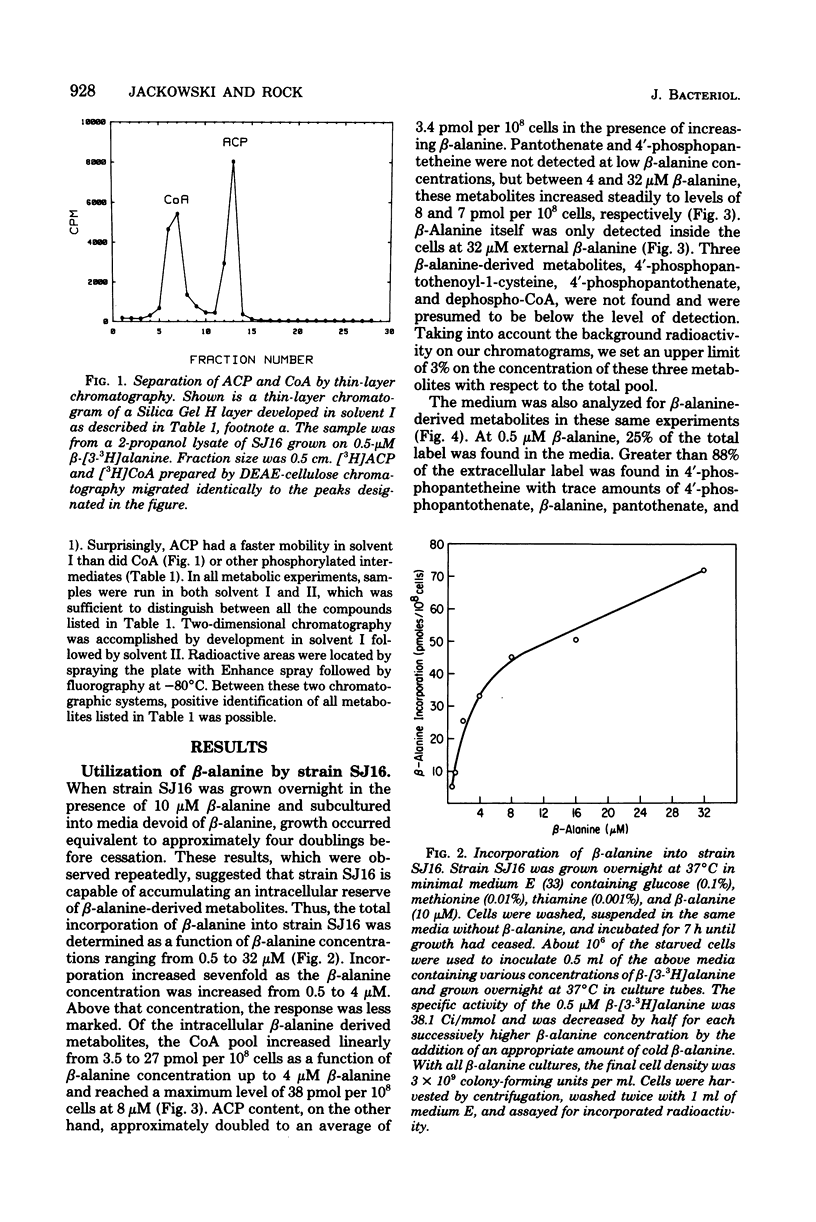
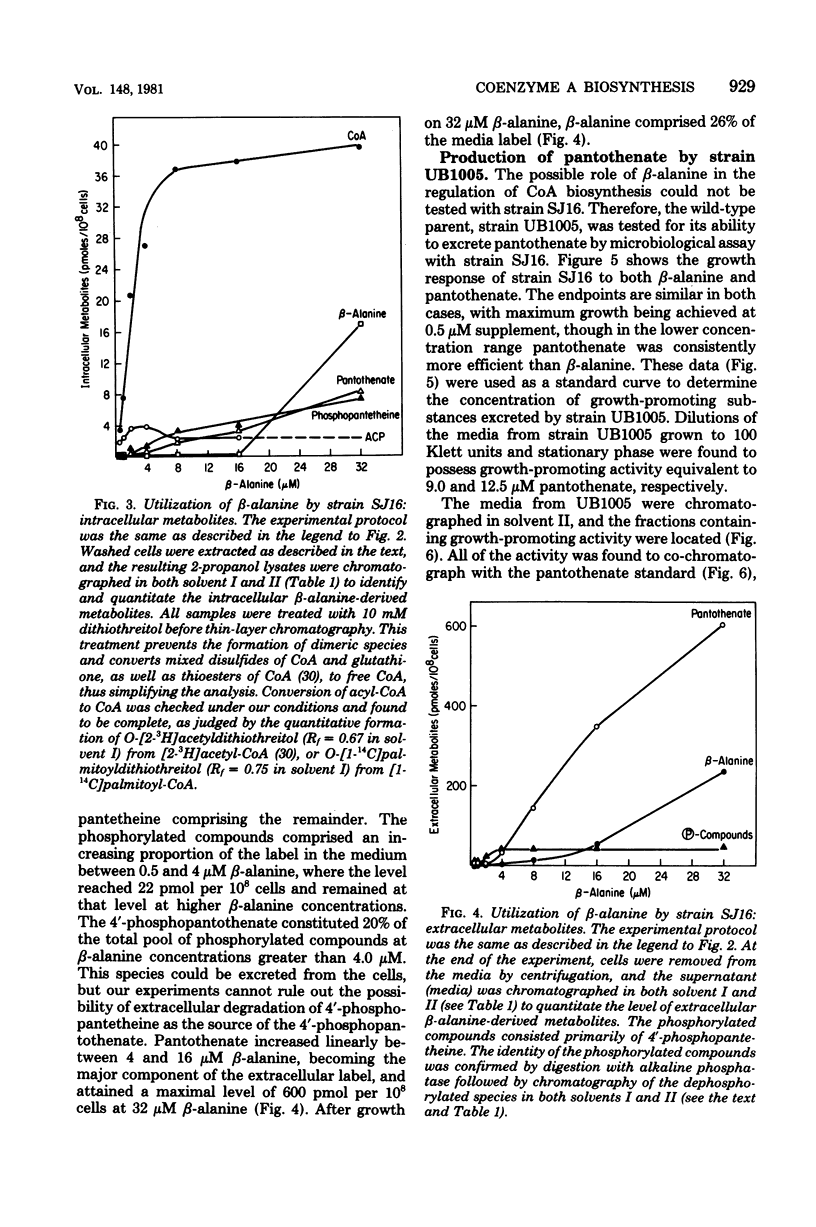
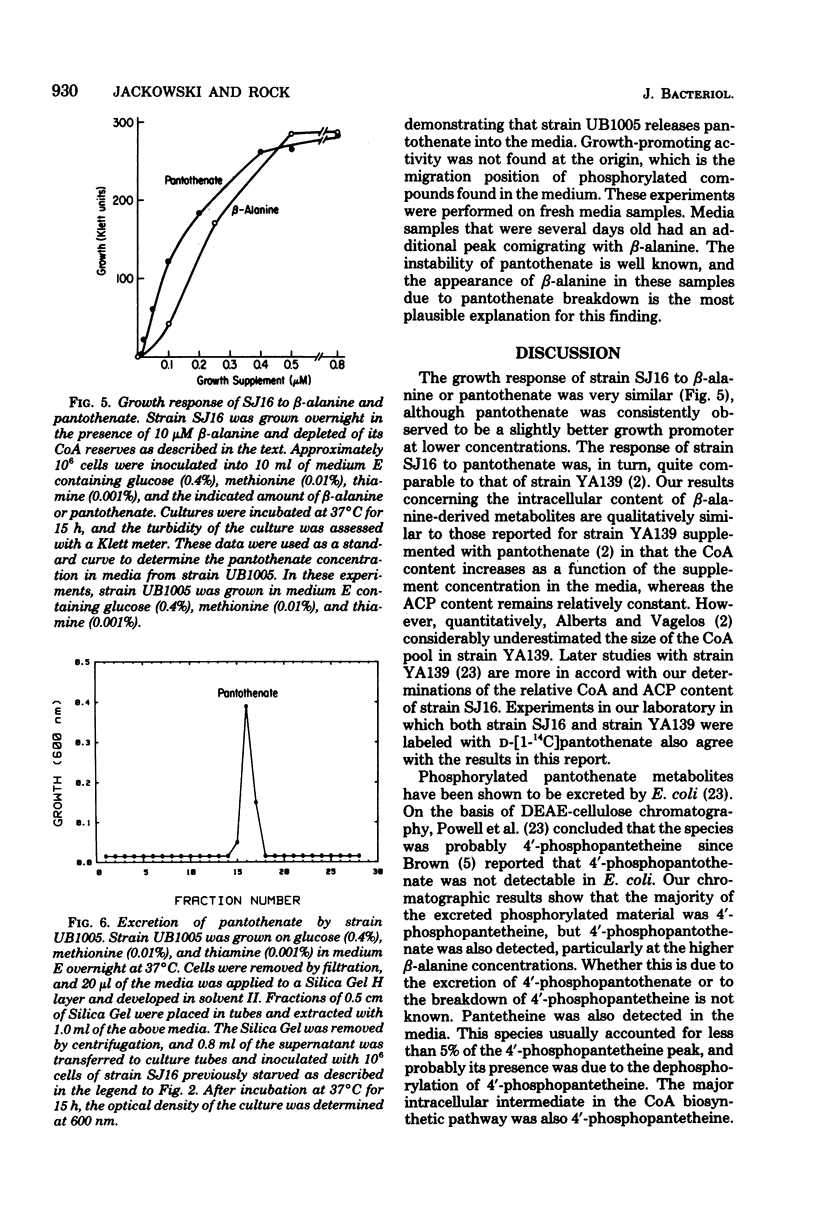
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiko Y., Ashida S. I., Shimizu M. Purification and properties of D-pantothenate kinase from rat liver. Biochim Biophys Acta. 1972 May 12;268(2):364–372. doi: 10.1016/0005-2744(72)90331-2. [DOI] [PubMed] [Google Scholar]
- Alberts A. W., Vagelos P. R. Acyl carrier protein. 8. Studies of acyl carrier protein and coenzyme A in Escherichia coli pantothenate or betaalanine auxotrophs. J Biol Chem. 1966 Nov 25;241(22):5201–5204. [PubMed] [Google Scholar]
- BROWN G. M. Assay and distribution of bound forms of pantothenic acid. J Biol Chem. 1959 Feb;234(2):379–382. [PubMed] [Google Scholar]
- BROWN G. M. The metabolism of pantothenic acid. J Biol Chem. 1959 Feb;234(2):370–378. [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock J. F., Pramanik A., Schulz H. Isolation of a multi-enzyme complex of fatty acid oxidation from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):492–495. doi: 10.1073/pnas.74.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. N., Sobhy C., Jenik R. A., Porter J. W. Interconversion of apo- and holofatty acid synthetases of rat and pigeon liver. Methods Enzymol. 1979;62:249–262. doi: 10.1016/0076-6879(79)62226-7. [DOI] [PubMed] [Google Scholar]
- Clark D. P., Cronan J. E., Jr Acetaldehyde coenzyme A dehydrogenase of Escherichia coli. J Bacteriol. 1980 Oct;144(1):179–184. doi: 10.1128/jb.144.1.179-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D., Cronan J. E., Jr Escherichia coli mutants with altered control of alcohol dehydrogenase and nitrate reductase. J Bacteriol. 1980 Jan;141(1):177–183. doi: 10.1128/jb.141.1.177-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr Beta-alanine synthesis in Escherichia coli. J Bacteriol. 1980 Mar;141(3):1291–1297. doi: 10.1128/jb.141.3.1291-1297.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovson J., Vagelos P. R. Acyl carrier protein. X. Acyl carrier protein synthetase. J Biol Chem. 1968 Jul 10;243(13):3603–3611. [PubMed] [Google Scholar]
- Karasawa T., Yoshida K., Furukawa K., Hosoki K. Feedback inhibition of pantothenate kinase by coenzyme A and possible role of the enzyme for the regulation of cellular coenzyme A level. J Biochem. 1972 Jun;71(6):1065–1067. doi: 10.1093/oxfordjournals.jbchem.a129854. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Loewen P. C. Levels of coenzyme A--glutathione mixed disulfide in Escherichia coli. Can J Biochem. 1978 Jul;56(7):753–759. doi: 10.1139/o78-113. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Pantothenate studies. III. Description of the extracted pantothenate-synthesizing enzyme of Escherichia coli. J Biol Chem. 1952 Sep;198(1):23–32. [PubMed] [Google Scholar]
- MICHELSON A. M. SYNTHESIS OF COENZYME A. Biochim Biophys Acta. 1964 Oct 9;93:71–77. doi: 10.1016/0304-4165(64)90261-2. [DOI] [PubMed] [Google Scholar]
- Miyatake K., Nakano Y., Kitaoka S. Pantothenate synthetase from Escherichia coli [D-pantoate: beta-alanine ligase (AMP-forming), EC 6.3.2.1]. Methods Enzymol. 1979;62:215–219. doi: 10.1016/0076-6879(79)62221-8. [DOI] [PubMed] [Google Scholar]
- O'Brien W. J., Frerman F. E. Evidence for a complex of three beta-oxidation enzymes in Escherichia coli: induction and localization. J Bacteriol. 1977 Nov;132(2):532–540. doi: 10.1128/jb.132.2.532-540.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. L., Elovson J., Vagelos P. R. Acyl carrier protein. XII. Synthesis and turnover of the prosthetic group of acyl carrier protein in vivo. J Biol Chem. 1969 Oct 25;244(20):5616–5624. [PubMed] [Google Scholar]
- Powers S. G., Snell E. E. Ketopantoate hydroxymethyltransferase. II. Physical, catalytic, and regulatory properties. J Biol Chem. 1976 Jun 25;251(12):3786–3793. [PubMed] [Google Scholar]
- Pugh E. L., Wakil S. J. Studies on the mechanism of fatty acid synthesis. XIV. The prosthetic group of acyl carrier protein and the mode of its attachment to the protein. J Biol Chem. 1965 Dec;240(12):4727–4733. [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr, Armitage I. M. Molecular properties of acyl carrier protein derivatives. J Biol Chem. 1981 Mar 25;256(6):2669–2674. [PubMed] [Google Scholar]
- Rock C. O., Garwin J. L. Preparative enzymatic synthesis and hydrophobic chromatography of acyl-acyl carrier protein. J Biol Chem. 1979 Aug 10;254(15):7123–7128. [PubMed] [Google Scholar]
- Stokes G. B., Stumpf P. K. Fat metabolism in higher plants. The nonenzymatic acylation of dithiothreitol by acyl coenzyme A. Arch Biochem Biophys. 1974 Jun;162(2):638–648. doi: 10.1016/0003-9861(74)90226-4. [DOI] [PubMed] [Google Scholar]
- Teller J. H., Powers S. G., Snell E. E. Ketopantoate hydroxymethyltransferase. I. Purification and role in pantothenate biosynthesis. J Biol Chem. 1976 Jun 25;251(12):3780–3785. [PubMed] [Google Scholar]
- Wilken D. R., King H. L., Jr, Dyar R. E. Ketopantoic acid and ketopantoyl lactone reductases. Stereospecificity of transfer of hydrogen from reduced nicotinamide adenine dinucleotide phosphate. J Biol Chem. 1975 Mar 25;250(6):2311–2314. [PubMed] [Google Scholar]
- Williamson J. M., Brown G. M. Purification and properties of L-Aspartate-alpha-decarboxylase, an enzyme that catalyzes the formation of beta-alanine in Escherichia coli. J Biol Chem. 1979 Aug 25;254(16):8074–8082. [PubMed] [Google Scholar]



