Abstract
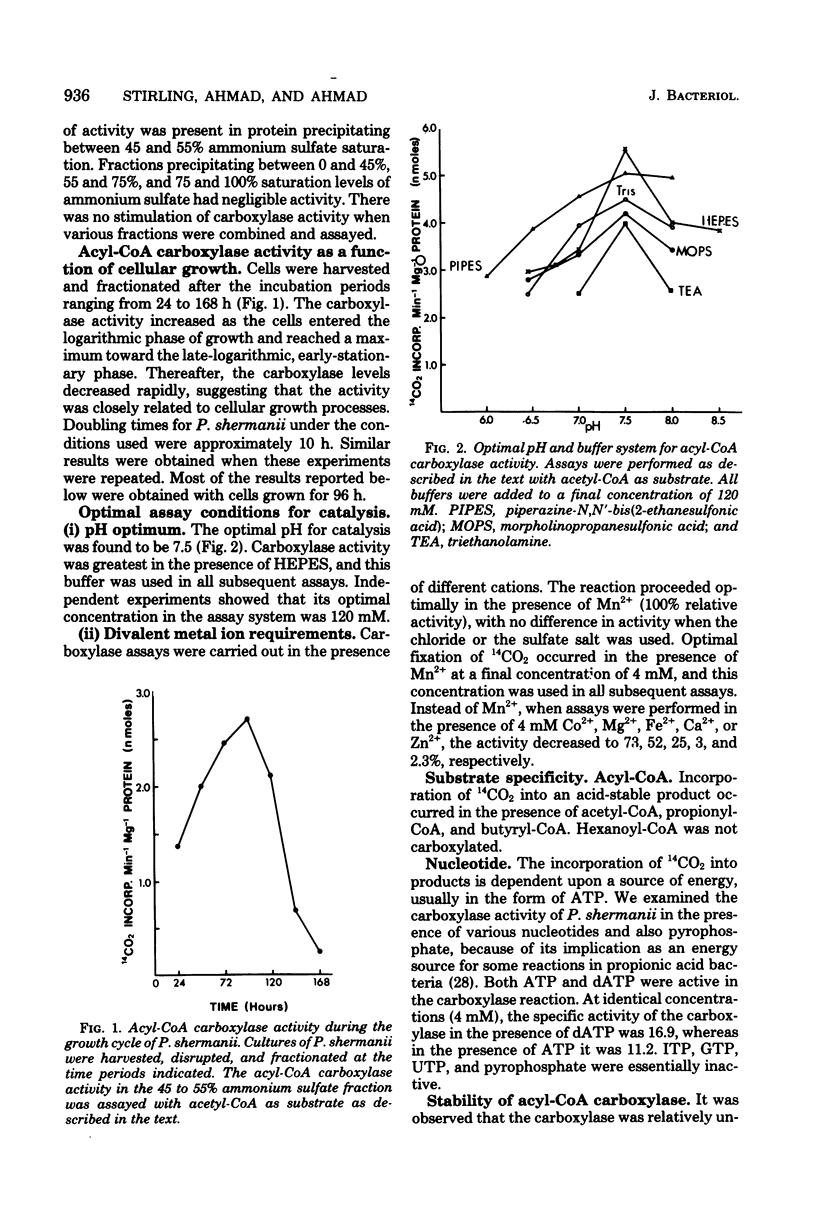
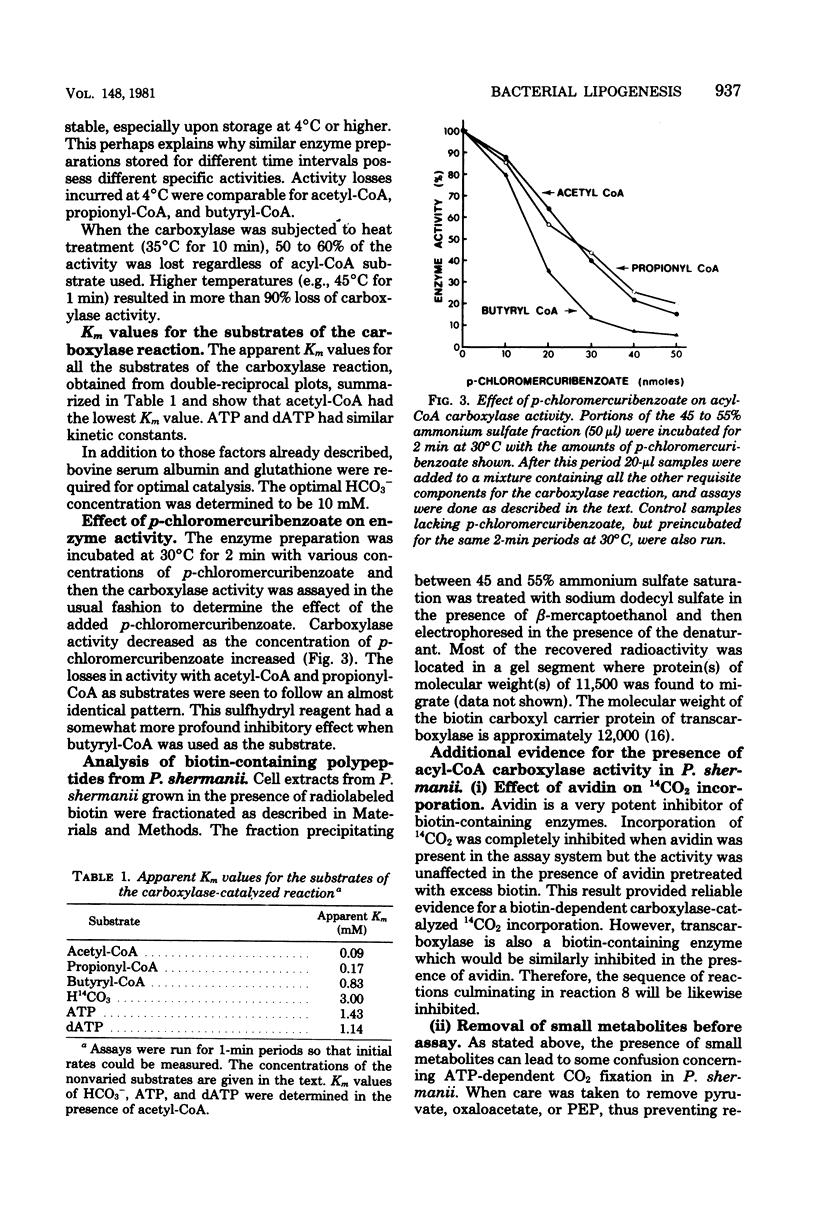
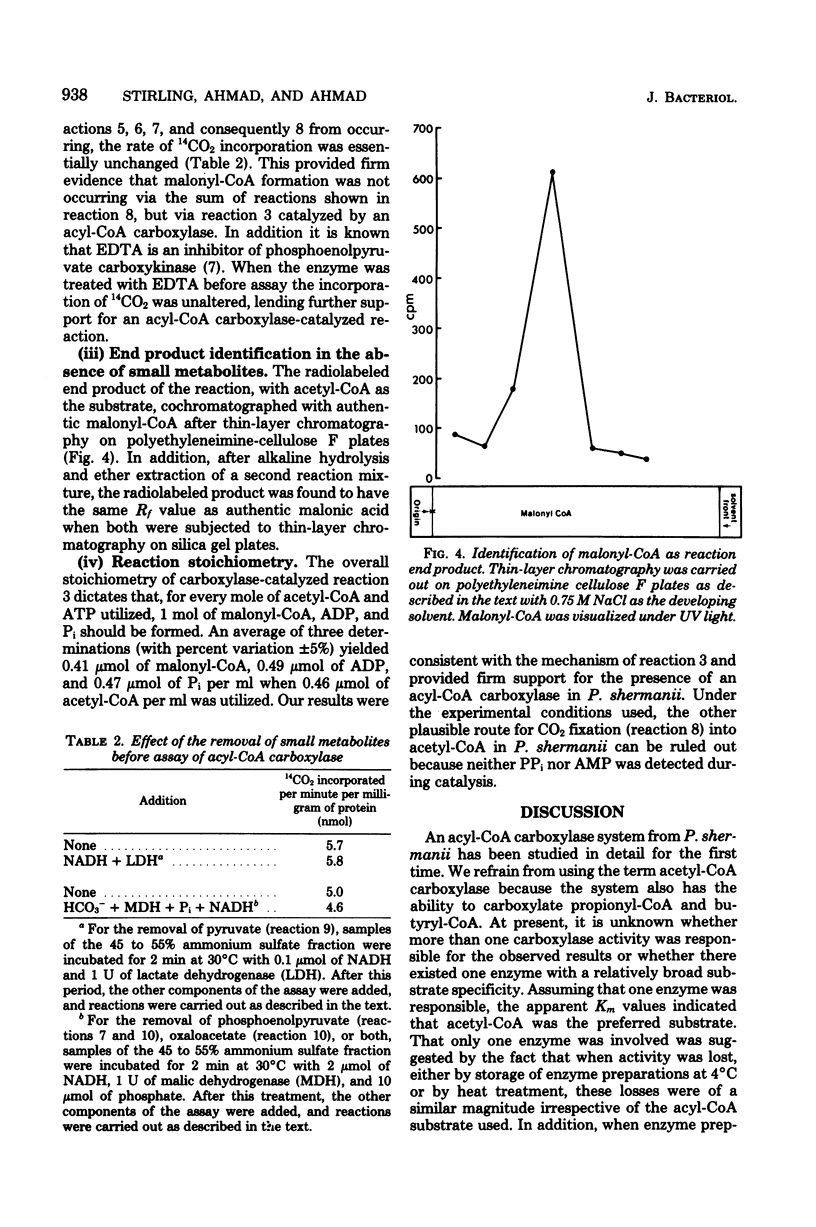
An acyl coenzyme A (CoA) carboxylase, which catalyzes the adenosine triphosphate-dependent fixation of CO2 into acetyl-, propionyl-, and butyryl-CoA, was detected in fractionated cell extracts of Propionibacterium shermanii. Catalytic activity was inhibited by avidin but was unaffected by avidin pretreated with excess biotin. The carboxylase levels detected were relatively small and were related to cellular growth. Maximal carboxylase activity was detected in cells grown for about 96 h. Thereafter, the activity declined rapidly. Optimal CO2 fixation occurred at pH 7.5. Other parameters of the assay system were optimized, and the apparent Km values for substrates were determined. The end product of the reaction (with acetyl-CoA as the substrate) was identified as malonyl-CoA. The stoichiometry of the reaction was such that, for every mole of acetyl-CoA and adenosine triphosphate consumed, 1 mol each of malonyl-CoA, adenosine diphosphate, and orthophosphate was formed. These data provide the first evidence for the presence of another biotin-containing enzyme, an acyl-CoA carboxylase, in these bacteria in addition to the well-characterized methylmalonyl-CoA carboxyltransferase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Nervi A. M., Vagelos P. R. Acetyl CoA carboxylase, II. Deomonstration of biotin-protein and biotin carboxylase subunits. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1319–1326. doi: 10.1073/pnas.63.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W., Vagelos P. R. Acetyl CoA carboxylase. I. Requirement for two protein fractions. Proc Natl Acad Sci U S A. 1968 Feb;59(2):561–568. doi: 10.1073/pnas.59.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davis J. J., Willard J. M., Wood H. G. Phosphoenolpyruvate carboxytransphosphorylase. 3. Comparison of the fixation of carbon dioxide and the conversion of phosphenolpyruvate and phosphate into pyruvate and pyrophosphate. Biochemistry. 1969 Aug;8(8):3127–3136. doi: 10.1021/bi00836a001. [DOI] [PubMed] [Google Scholar]
- Delwiche E. A. VITAMIN REQUIREMENTS OF THE GENUS PROPIONIBACTERIUM. J Bacteriol. 1949 Sep;58(3):395–398. doi: 10.1128/jb.58.3.395-398.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfle J. D. Acetyl-CoA and propionyl-CoA carboxylation by Mycobacterium phlei. Partial purification and some properties of the enzyme. Biochim Biophys Acta. 1973 Aug 23;316(2):143–155. doi: 10.1016/0005-2760(73)90004-0. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Wood H. G. The mechanism of the pyruvate, phosphate dikinase reaction. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1448–1453. doi: 10.1073/pnas.61.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R. R., Alberts A. W., Vagelos P. R. Analysis of bacterial biotin-proteins. Biochim Biophys Acta. 1975 Feb 27;379(2):496–503. doi: 10.1016/0005-2795(75)90156-7. [DOI] [PubMed] [Google Scholar]
- Fall R. R., Nervi A. M., Alberts A. W., Vagelos P. R. Acetyl CoA carboxylase: isolation and characterization of native biotin carboxyl carrier protein. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1512–1515. doi: 10.1073/pnas.68.7.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R. R. Stabilization of an acetyl-coenzyme A carboxylase complex from Pseudomonas citronellolis. Biochim Biophys Acta. 1976 Dec 20;450(3):475–480. doi: 10.1016/0005-2760(76)90022-9. [DOI] [PubMed] [Google Scholar]
- Fall R. R., Vagelos P. R. Acetyl coenzyme A carboxylase. Molecular forms and subunit composition of biotin carboxyl carrier protein. J Biol Chem. 1972 Dec 25;247(24):8005–8015. [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Jacobson B. E., Wood H. G. Transcarboxylase. 8. Isolation and properties of a biotin-carboxyl carrier protein. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1315–1322. doi: 10.1073/pnas.64.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guchhait R. B., Polakis S. E., Dimroth P., Stoll E., Moss J., Lane M. D. Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and properties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J Biol Chem. 1974 Oct 25;249(20):6633–6645. [PubMed] [Google Scholar]
- Hector M. L., Fall R. R. Multiple acyl-coenzyme A carboxylases in Pseudomonas citronellolis. Biochemistry. 1976 Aug 10;15(16):3465–3472. doi: 10.1021/bi00661a011. [DOI] [PubMed] [Google Scholar]
- Hess H. H., Derr J. E. Assay of inorganic and organic phosphorus in the 0.1-5 nanomole range. Anal Biochem. 1975 Feb;63(2):607–613. doi: 10.1016/0003-2697(75)90388-7. [DOI] [PubMed] [Google Scholar]
- Lane M. D., Moss J., Polakis S. E. Acetyl coenzyme A carboxylase. Curr Top Cell Regul. 1974;8(0):139–195. [PubMed] [Google Scholar]
- Lynen F. New experiments of biotin enzymes. CRC Crit Rev Biochem. 1979 Dec;7(2):103–119. doi: 10.3109/10409237909105428. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Levy H. R. Rat mammary acetyl coenzyme A carboxylase. I. Isolation and characterization. J Biol Chem. 1969 May 10;244(9):2334–2342. [PubMed] [Google Scholar]
- SIU P. M., WOOD H. G. Phosphoenolpyruvic carboxytransphosphorylase, a CO2 fixation enzyme from propionic acid bacteria. J Biol Chem. 1962 Oct;237:3044–3051. [PubMed] [Google Scholar]
- Scrutton M. C., Utter M. F. Pyruvate carboxylase. V. Interaction of the enzyme with adenosine triphosphate. J Biol Chem. 1965 Oct;240(10):3714–3723. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]
- Wood H. G. Some reactions in which inorganic pyrophosphate replaces ATP and serves as a source of energy. Fed Proc. 1977 Aug;36(9):2197–2206. [PubMed] [Google Scholar]


