Abstract
The myxobacterium Stigmatella aurantiaca passes through a life cycle that involves formation of a multicellular fruiting body as the most complex stage. An early step in this differentiation process depends on a signal factor secreted by the cells when nutrients become limited. The formation of a fruiting body from a small cell population can be accelerated by addition of this secreted material. The bioactive compound was found to be steam volatile. It was purified to homogeneity by steam distillation followed by reversed-phase and normal-phase HPLC. The pheromone was named stigmolone, in accordance with the structure 2,5,8-trimethyl-8-hydroxy-nonan-4-one, as determined by NMR and mass spectrometry. Stigmolone represents a structurally unique and highly bioactive prokaryotic pheromone that is effective in the bioassay at 1 nM concentration.
Keywords: microbial development/differentiation/fruiting bodies
Stigmatella aurantiaca is a myxobacterium, which lives in swarms on solid surfaces. As is typical for myxobacteria, this organism survives periods of starvation by undergoing a developmental process in which the individuals of a swarm aggregate to form multicellular fruiting bodies. These species-specific fruiting bodies harbor the myxospores, which are dormant cells. The morphologically complex fruiting body of S. aurantiaca has a treelike structure, and spores are packaged in sporangioles located on the top of the structure. When the supply of nutrients increases (which also may occur after dissemination of the sporangioles), the myxospores of a sporangiole germinate to build up a new swarm of vegetative cells. Under favorable conditions the growth of the swarm is based on transverse binary fission of the cells (1–3).
The switch from the socially living single cells to the multicellular fruiting body, which contains a variety of differentiated cells, is a prokaryotic paradigm for differentiation processes and signal transduction. To build up the fruiting body as an elaborate and spatially complex multicellular structure, the cells must exchange signals during the entire process. For the related organism Myxococcus xanthus several of the genes and developmental signals involved in fruiting body formation have been characterized, as summarized in a review (1).
In 1982 Stephens et al. (4) demonstrated a pheromone activity in an aqueous eluate obtained from filter paper on which S. aurantiaca had formed fruiting bodies. This activity also was found in chloroform extracts of the eluate, but purification of active substances was not performed. In this paper we demonstrate that specific signal(s) trigger the aggregation of cells, a step that is essential for the initiation of the differentiation process for S. aurantiaca. We describe the purification of a hydroxy ketone with the formula C12H24O2, named stigmolone, which represents a novel type of prokaryotic signal molecule required for the developmental cycle of S. aurantiaca. The structure of this compound, 2,5,8-trimethyl-8-hydroxy-nonan-4-one, was determined by NMR and mass spectrometry (5).
MATERIALS AND METHODS
Chemicals.
Solvents used for HPLC were: LiChrosolv water (Merck), acetonitrile (ultragradient grade, J. T. Baker), methyl acetate (Fluka), and cyclopentane (Aldrich).
Bacterial Strains and Growth Conditions.
S. aurantpaca DW4/3–1 (6) was grown at pH 7.2 and 32°C in 1% bacto tryptone (Difco), supplemented with 8 mM MgSO4 and 120 mg/liter streptomycin sulfate. Cultures were aerated by gyration (up to 800 ml) or grown in a 10-liter fermenter (ISF 200, Infors AG, Bottmingen, Switzerland) at 95% pO2 and constant pH. Myxococcus xanthus DK1622 (7) was grown in CTT medium (1% casitone, 8 mM MgSO4, 10 mM Tris⋅HCl, 1 mM potassium phosphate, pH 7.6) (8) at 32°C.
Bioassay.
The assay of pheromone activity was performed according to Stephens et al. (4). A 200-ml culture of S. aurantiaca (4 × 1010 cells) was harvested by centrifugation (15 min., 4,200 g, 4°C), washed at 4°C with 200 ml of 10 mM CaCl2 in 100 mM Hepes/NaOH, pH 7.2, and finally suspended in this buffer to a density of 1 × 1010 cells/ml. Samples of 1-ml volume containing the fractions to be tested were supplemented with 1.5% agar (Difco), 7.8 mM CaCl2, 10 mM Hepes/NaOH, pH 7.2, and placed in wells of a 24-well tissue culture plate (Greiner, Nürtingen, Germany) at 60°C. The solidified agar was dried for 20 min in a hood, and the cell suspension was spotted in portions of 5 μl (5 × 107 cells) onto the agar. After the spots had dried, the covered plates were incubated at 32°C in the dark. The appearance of aggregates and fruiting bodies was monitored after 10–20 hr.
The assay conditions for M. xanthus DK1622 were adapted as follows. Cells of a culture (4 × 107 cells/ml) were harvested and washed as described above. The final suspension was adjusted to 8 × 108 cells/ml and spotted in portions of 5 μl onto the agar containing up to 0.2 mM Stigmatella pheromone. The appearance of aggregates and fruiting bodies was monitored after 12–24 hr.
Dialysis Experiment.
A dialysis membrane (from a Visking dialysis tube, exclusion limit 8,000–15,000 dalton) was mounted in a microdialysis apparatus (model 1200MD, Bethesda Research Laboratories) with 6.8 mM CaCl2 in buffer wells on the lower side of the membrane and air on the upper side. Aliquots of 3 × 108 cells (5 μl) per well were placed on the membrane and dried. Intensive dialysis was achieved by pumping the calcium chloride solution through the buffer wells; the content of the wells was mixed by permanent stirring. Dialysis was performed at 32°C under fluorescent light (ca. 500 lx). The possible influence of light intensity was not investigated in this study, but see ref. 9 for effects of light on Stigmatella development.
Large-Scale Production of the Pheromone.
Cells of a 10-liter culture (2 × 1012 cells) were harvested by tangential filtration using a Pellicon 0.45-μm cassette (Millipore) and centrifugation (4,200 g, 4°C). The cells were washed at 4°C with 700 ml of 10 mM CaCl2 in 100 mM Hepes/NaOH, pH 7.2, and finally suspended in ca. 50 ml buffer to a density of 4 × 1010 cells per ml. This suspension was spotted in 5-μl portions on 7.5 × 30 cm filter paper strips (Whatman 3MM) moistened with 6.8 mM CaCl2 at a density of about 1.8 spots/cm2. The paper strips were eluted in a chamber for descending paper chromatography under white fluorescent light (ca. 500 lx) using 6.8 mM CaCl2 as descending solvent. The eluate from the strips was collected over a period of 130 hr (about 3 liters from 2 × 1012 cells) and stored at −20°C.
Steam Distillation.
The paper eluate (1.7 liters) was heated in a two-liter flask, and steam from an external generator was blown through the sample. The condensed distillate (1.7 l) was stored at −20°C. Up to 22 liters of such distillates were pumped through a glass column (2.5-cm diameter) containing 10 g of C18 solid-phase extraction resin (Chromabond C18, Macherey & Nagel). The bound pheromone was recovered in 14 ml by elution with 60% CH3CN.
Purification by HPLC.
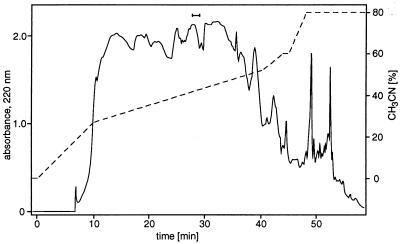
Up to 30 ml of the concentrated pheromone solution were diluted with 9 vol of water. The solution was loaded in successive 5-ml portions (1 ml/min) onto a 250 × 4.6-mm C18 column (Spherisorb, 5 μm ODSII; Kontron Instruments, Neufahrn, Germany) and fractionated by reversed-phase HPLC (Kontron) at 1 ml/min by using a CH3CN gradient generated by mixing 10 mM acetic acid/NaOH, pH 5.0 with 80% (vol/vol) CH3CN in water (see Fig. 3). Fractions were collected at 40-s intervals and assayed for activity.
Figure 3.
Purification of the pheromone by reversed-phase HPLC. A total of 220 ml of dilute pheromone solution obtained by steam distillation (derived from ca. 2.5 × 1013 cells) was loaded in successive 5-ml portions onto a C18 column (250 × 4.6 mm, 5 μm) at 1 ml/min. Pheromone was eluted at 1 ml/min with a gradient (dashed line) of CH3CN in acetate buffer. Fractions were collected, and aliquots were assayed for pheromone activity. The biologically active fractions at a retention time of ca. 28 min are indicated by a bar.
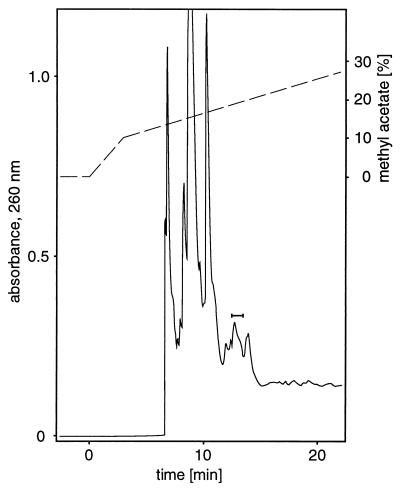
The active fractions from several reversed-phase HPLC runs, comprising the processed material collected from about 8 × 1013 starving cells, were combined (6.4 ml), diluted with 11 vol of water, and loaded onto two 1-ml glass solid-phase extraction columns containing 100 mg Bakerbond spe* C18 (J. T. Baker). The columns were dried in a vacuum to constant weight, and the pheromone was recovered by elution with dry, redistilled diethyl ether. After evaporation of the ether by a stream of dry nitrogen at room temperature the residue was dissolved in cyclopentane. The cyclopentane solution containing the processed material from up to 4 × 1013 starving cells was fractionated by normal-phase HPLC using an amino propyl column (Nucleosil NH2, 10 μm, 250 × 4 mm; Macherey-Nagel) (see Fig. 4). Fractions were collected at 30-s intervals and assayed for activity.
Figure 4.
Purification of the pheromone by normal-phase HPLC. A cyclopentane solution of pheromone (550 μl; derived from ca. 4 × 1013 cells and prepurified by reversed-phase HPLC) was loaded onto a Nucleosil NH2 column using a 1-ml sample loop and eluted at 1 ml/min with a gradient (dashed line) of methyl acetate in cyclopentane. Fractions with pheromone activity are indicated by a bar.
For spectroscopic analyses, for example, it was necessary to transfer the purified pheromone into other solvents. For this purpose the solvent mixture of cyclopentane and methyl acetate was removed from the active HPLC fractions by a stream of dry nitrogen at room temperature without loss of the pheromone.
RESULTS
Bioassay.
Aggregation (and consequently fruiting body formation) of a low-density population of S. aurantiaca cells was accelerated by addition of pheromone samples to the agar on which the cells were placed. This acceleration was used as an assay for pheromone activity (4) and was quantified as the number of aggregates or fruiting bodies observed after a specified time period with a precision of ca. ±15%. Cells in control experiments (without any added pheromone) usually developed aggregates only after about 20 hr at the earliest; the addition of pheromone reduced this time by up to 10 hr.
Proof of the Pheromone Requirement by Dialysis.
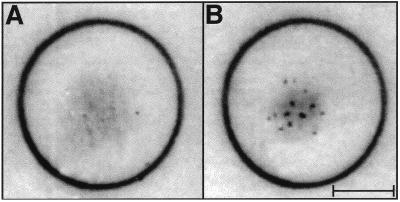
The following experiment demonstrated unequivocally the essential role of secreted extracellular factor(s) for aggregation and the differentiation process under starvation conditions. S. aurantiaca cells were placed on a dialysis membrane (Fig. 1), and during dialysis against 6.8 mM CaCl2 (starvation), no aggregation took place. If dialysis was stopped after 24 hr, aggregates began to form. In this case the time required for aggregation was shorter than in a control experiment performed without dialysis pretreatment. This difference is most likely caused by the time lag observed for controls between the initiation of starvation conditions and the beginning of pheromone secretion (see below and Fig. 2).
Figure 1.
Demonstration that a diffusable pheromone is required for aggregation of starved S. aurantiaca cells. (A) Cells (3 × 108) on a dialysis membrane were dialyzed for 24 hr against 6.8 mM CaCl2 at 32°C. No aggregation of the cells was observed. (B) After an additional 24 hr at 32°C without dialysis, aggregates of cells have formed on the dialysis membrane shown in A. The calibration bar corresponds to 5 mm. The result (not shown) of a control experiment (cells placed on a dialysis membrane for 24 hr, but without dialysis) was indistinguishable from B.
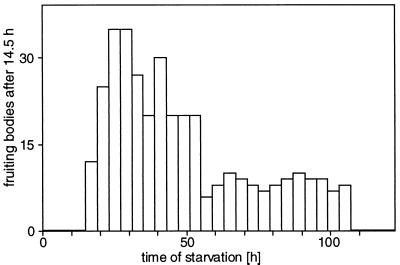
Figure 2.
Time course of pheromone elution in the large-scale production system. A filter paper strip spotted with 8.4 × 1010 adhering S. aurantiaca cells was placed in a conventional descending paper chromatography chamber and eluted with 6.8 mM CaCl2. Fractions were collected every 4 hr, and 50-μl aliquots were assayed directly for pheromone activity, which was quantified as the number of fruiting bodies observed after 14.5 hr.
Large-Scale Production of the Pheromone.
Several attempts to obtain pheromone produced in suspension cultures of S. aurantiaca cells were undertaken, but all failed. The secretion of the pheromone may depend on the contact of the cells with a solid surface or with each other. Therefore, we took advantage of the fact that S. aurantiaca cells stick tightly to filter paper. In a conventional paper chromatography chamber cells on strips of filter paper remained in place even with a descending flow of 6.8 mM CaCl2 through the strips while all soluble, secreted molecules were eluted continuously from the paper. The time course for the appearance of pheromone activity in the eluate is given in Fig. 2. During the first 15 hr pheromone was not detectable in the eluate by the bioassay (as defined in Fig. 2). The pheromone concentration reached a maximum about 24 hr after starvation began and decreased to a plateau level at ca. 60 hr. After about 5 days pheromone secretion ceased, probably because of the effects of long-term starvation. During elution the cells on the filter paper were unable to form aggregates, analogous to the behavior seen in the dialysis experiment described above.
Purification of the Pheromone.
Several attempts to concentrate the crude pheromone-containing solution under reduced pressure resulted in a substantial loss of pheromone activity. The pheromone was chemically stable under the conditions used but was found to be volatile. By using steam distillation all nonvolatile contaminants could be removed, and quantitative recovery of the pheromone was possible if a volume equal to the sample volume was collected as condensate.
The pheromone was concentrated by passing the steam distillate through a solid-phase extraction column. The adsorbed pheromone then was reclaimed by elution with 60% acetonitrile in water, and the recovered yield was greater than 80%. This eluate was diluted with water and loaded onto a reversed-phase HPLC column. A gradient of CH3CN in acetate buffer was used to obtain elution of the pheromone activity at about 42% CH3CN. Nearly the entire activity was recovered in a narrow time interval as indicated in Fig. 3.
Because of the volatility of the pheromone, it was not possible to evaporate the solvent from the active fractions without nearly complete loss of the active material. Therefore, the material was bound to a C18 solid-phase extraction resin, and water was removed by drying the resin to constant weight without loss of the active material. The pheromone activity was eluted by dry diethyl ether because elution with cyclopentane failed. After evaporation of the ether the residue was dissolved in cyclopentane, with a recovery of better than 80%, and loaded onto a Nucleosil NH2 column for normal-phase HPLC. Elution was achieved with a linear gradient of methyl acetate in cyclopentane as indicated in Fig. 4. The recovery for this step was greater than 80%, and again the activity was detected in a narrow time interval.
Analysis of the Purified Pheromone.
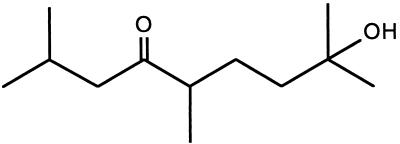
Material secreted from 8 × 1013 cells was processed to yield finally about 2 μmol of purified pheromone. The pooled active fractions from the normal-phase HPLC were analyzed by mass spectrometry (MS) and gas chromatography. Only one single GC peak was detected (data not shown) that displayed the same molecular fragments upon MS analysis as observed with the original sample. This finding suggested that the compound had been purified to homogeneity. MS using electrospray ionization gave m/z = 223.1 for the M+Na complex, indicating a molecular weight of 200 for the pheromone. High-resolution MS (electron-impact ionization) analysis of fragment ions at m/z = 182 (M − H2O) and 185 (M − CH3) gave a molecular formula of C12H24O2 for the parent molecule. Detailed 1H- and 13C-NMR analyses led to the identification of the pheromone as 2,5,8-trimethyl-8-hydroxy-nonan-4-one (Scheme S1), which was further confirmed by synthesis (5). Thus, the pheromone is an aliphatic hydroxy ketone, which we named stigmolone.
Scheme 1.
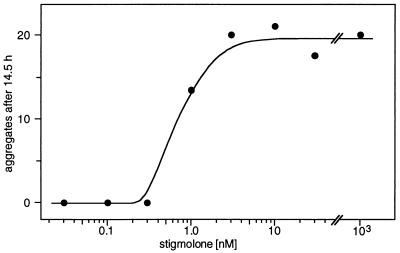
With pure stigmolone the threshold for detection in our bioassay was ca. 0.5–1 nM and maximal response was obtained at ca. 2 nM (Fig. 5). Extensive experiments with synthetic stigmolone (5) confirmed these results. The biological activity appears to be species specific; stigmolone did not accelerate the formation of fruiting bodies in low-density populations of M. xanthus (DK1622).
Figure 5.
Dose-response of S. aurantiaca to stigmolone. Data were obtained with our standard assay as the number of cell aggregates observed after 14.5 hr.
DISCUSSION
The purified signaling factor of S. aurantiaca is a true pheromone according to the definition given by Karlson and Lüscher (10), because it serves for communication between organisms of the same species. While searching for a purification procedure, we unexpectedly found stigmolone to be volatile. This property was ruled out in earlier reports (4, 11), but this conclusion may have been dictated by the specific experimental conditions.
Our continuous elution procedure for large-scale pheromone production has an advantage over the batch process described earlier (4) because all secreted pheromone is collected. In the batch process the cells were allowed to build fruiting bodies on a filter paper stack coated with a thin agar layer. The top sheet then was discarded, and the pheromone was eluted from only the lower sheets. Furthermore, with our continuous elution protocol probably more pheromone per cell is secreted because pheromone is continuously removed and aggregation of the cells is arrested.
During the last 45 years the involvement of pheromone-like activities in myxobacterial development has quite often been described or suggested. Corallococcus exiguus (formerly Chondrococcus) and M. xanthus were found to produce substances that stimulated the formation of fruiting bodies (12). Fruiting body formation by M. virescens could be stimulated by extracts from fruiting bodies of the same species (13). For M. xanthus and M. fulvus it was shown that fruiting bodies under a piece of cellophane could affect the location of fruiting bodies formed by a second layer of cells on the upper surface of the cellophane (14, 15). A pheromone activity for S. aurantiaca previously was ascribed to a “nonvolatile, low-molecular-weight lipid” or “lipoidal substance” (4, 11). The molecular weight and hydrophobicity of stigmolone are consistent with this description; however, stigmolone exhibits significant volatility from aqueous solutions. This contradiction might be alleviated by the reported observation of varying losses of pheromone activity during concentration and extraction procedures (4).
From a theoretical viewpoint the aggregation phenomenon of myxobacteria was attributed to a diffusible, cell density-dependent chemoattractant for the stabilization of preaggregation centers (16, 17). For M. fulvus and M. xanthus chemotaxis during fruiting body formation has been described (14, 15). Methylation of the chemotaxis protein FrzCD in M. xanthus was observed to depend on cell density (18). The importance of cell density sensing is further substantiated by the observation that there is a lower limit to the number of cells that must be present within a certain area for fruiting body formation to occur (19). For M. xanthus adenosine and the A-signal (an extracellular amino acid mixture) are involved in cell-density sensing (20, 21), whereas the E-signal (branched-chain fatty acids) participates in cell–cell communication (22). Apart from adenosine, the A-signal, and the E-signal, no other low-molecular-weight signal molecule of myxobacteria has been purified or chemically characterized up to now.
The observed inability of Stigmatella cells to aggregate during dialysis leads to the conclusion that at least one essential pheromone is required for aggregation, the early step in fruiting body formation. Stigmolone appears to be a good candidate for this function as both natural and synthetic stigmolone have demonstrated their ability to accelerate aggregation of starving cells. Stigmolone also may be involved in other aspects of the differentiation processes mentioned above.
If crude material (for instance the eluate from filter paper) produced from 2 × 108 starving cells during 24 hr was applied in a bioassay with 5 × 107 cells, the rate of aggregation was found to be comparable to that observed with a population of 2 × 108 cells under control conditions without addition of signal factor. Thus, the quantitative effect of a given amount of pheromone correlates with the number of cells that produced this amount. Therefore, it appears likely that stigmolone contributes to cell density sensing, which is involved in triggering the initiation of the developmental branch of the life cycle.
Stigmolone does not belong to any known class of pheromones or signal substances of other bacteria (23–26). The threshold concentration for an accelerating effect on aggregation was found to be ca. 1 nM, which makes this pheromone one of the most effective nonpeptidic bacterial pheromones known (26). For example, stigmolone’s potency is similar to that found for the most effective members of the well-known homoserine lactone group. For the distantly related eukaryotic system Dictyostelium discoideum, similar or higher concentrations are required for signaling with molecules such as cAMP, DIF-1, or ammonia (27, 28).
In the future we would like to identify the cellular control mechanisms involved in timing the synthesis and release of stigmolone and to elucidate the biosynthetic pathway itself. Such investigations may be easier with other myxobacteria species, which can be grown on defined, synthetic media. With the purification and analysis methods presented here, it should be possible to identify comparable signal factors in other myxobacteria, provided that these species also make use of related pheromones.
Acknowledgments
We gratefully acknowledge the help of Ludger Witte and Wolfram Schäfer with analyses at an early stage of this project. We also thank Dale Kaiser and David White for providing bacterial strains, Klaus Dörich for continuous maintenance of the fermenter, and William E. Hull for a critical reading of the manuscript.
References
- 1.Dworkin M. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dworkin M, Kaiser D, editors. Myxobacteria II. Washington, DC: Am. Soc. Microbiol.; 1993. [Google Scholar]
- 3.Shimkets L J. Microbiol Rev. 1990;54:473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens K, Hegeman G D, White D. J Bacteriol. 1982;149:739–747. doi: 10.1128/jb.149.2.739-747.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hull W E, Berkessel A, Plaga W. Proc Natl Acad Sci USA. 1998;95:11268–11273. doi: 10.1073/pnas.95.19.11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qualls G T, Stephens K, White D. Dev Biol. 1978;66:270–274. doi: 10.1016/0012-1606(78)90291-9. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser D. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgkin J, Kaiser D. Proc Natl Acad Sci USA. 1977;74:2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qualls G T, Stephens K, White D. Science. 1978;201:444–445. doi: 10.1126/science.96528. [DOI] [PubMed] [Google Scholar]
- 10.Karlson P, Lüscher M. Nature (London) 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 11.White D. Int Rev Cytol. 1981;72:203–227. [Google Scholar]
- 12.Lev M. Nature (London) 1954;173:501. [Google Scholar]
- 13.Jennings J. Nature (London) 1961;190:190. doi: 10.1038/190190b0. [DOI] [PubMed] [Google Scholar]
- 14.McVittie A, Zahler S A. Nature (London) 1962;194:1299–1300. [Google Scholar]
- 15.Fluegel W. Proc Minn Acad Sci. 1963;30:120–123. [Google Scholar]
- 16.Stevens A. In: Proceedings Conference on Nonlinear Wave Processes in Excitable Media. Holden A V, Markus M, Othmer H G, editors. New York: Plenum; 1991. pp. 269–276. [Google Scholar]
- 17.Stevens A. J Biol Syst. 1995;3:1059–1068. [Google Scholar]
- 18.Shi W, Ngok F K, Zusman D R. Proc Natl Acad Sci USA. 1996;93:4142–4146. doi: 10.1073/pnas.93.9.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wireman J W, Dworkin M. Science. 1975;189:516–523. doi: 10.1126/science.806967. [DOI] [PubMed] [Google Scholar]
- 20.Shimkets L J, Dworkin M. Dev Biol. 1981;84:51–60. doi: 10.1016/0012-1606(81)90369-9. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan H B, Plamann L. FEMS Microbiol Lett. 1996;139:89–95. doi: 10.1111/j.1574-6968.1996.tb08185.x. [DOI] [PubMed] [Google Scholar]
- 22.Downard J, Toal D. Mol Microbiol. 1995;16:171–175. doi: 10.1111/j.1365-2958.1995.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 23.Gray K M. Trends Microbiol. 1997;5:184–188. doi: 10.1016/S0966-842X(97)01002-0. [DOI] [PubMed] [Google Scholar]
- 24.Swift S, Bainton N J, Winson M K. Trends Microbiol. 1994;2:193–198. doi: 10.1016/0966-842x(94)90110-q. [DOI] [PubMed] [Google Scholar]
- 25.Swift S, Throup J P, Williams P, Salmond G P C, Stewart G S A B. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 26.Wirth R, Muscholl A, Wanner G. Trends Microbiol. 1996;4:96–103. doi: 10.1016/0966-842X(96)81525-3. [DOI] [PubMed] [Google Scholar]
- 27.Berks, M., Traynor, D., Carrin, I., Insall, R. H. & Kay, R. R. (1991) Development (Cambridge, U.K.), Suppl. 1, 131–139. [PubMed]
- 28.Gross J D. Microbiol Rev. 1994;58:330–351. doi: 10.1128/mr.58.3.330-351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]