Abstract
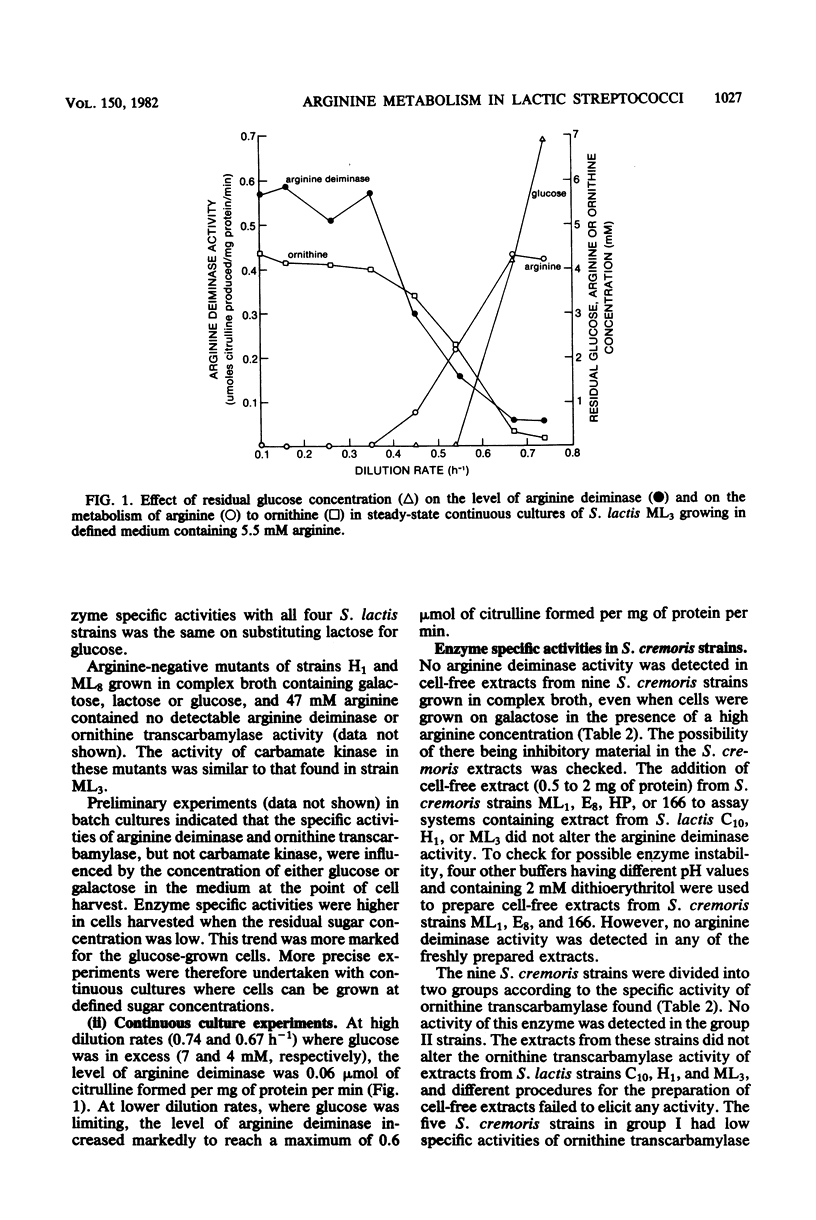
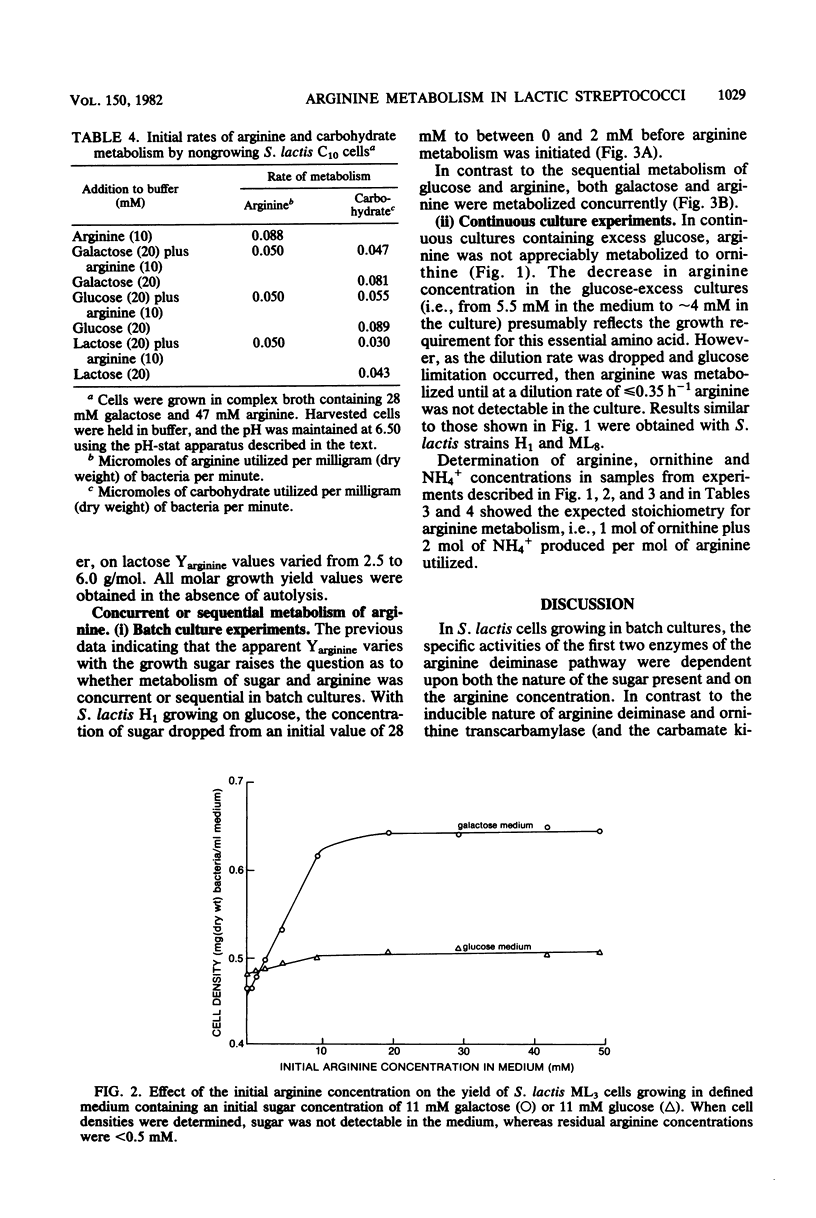
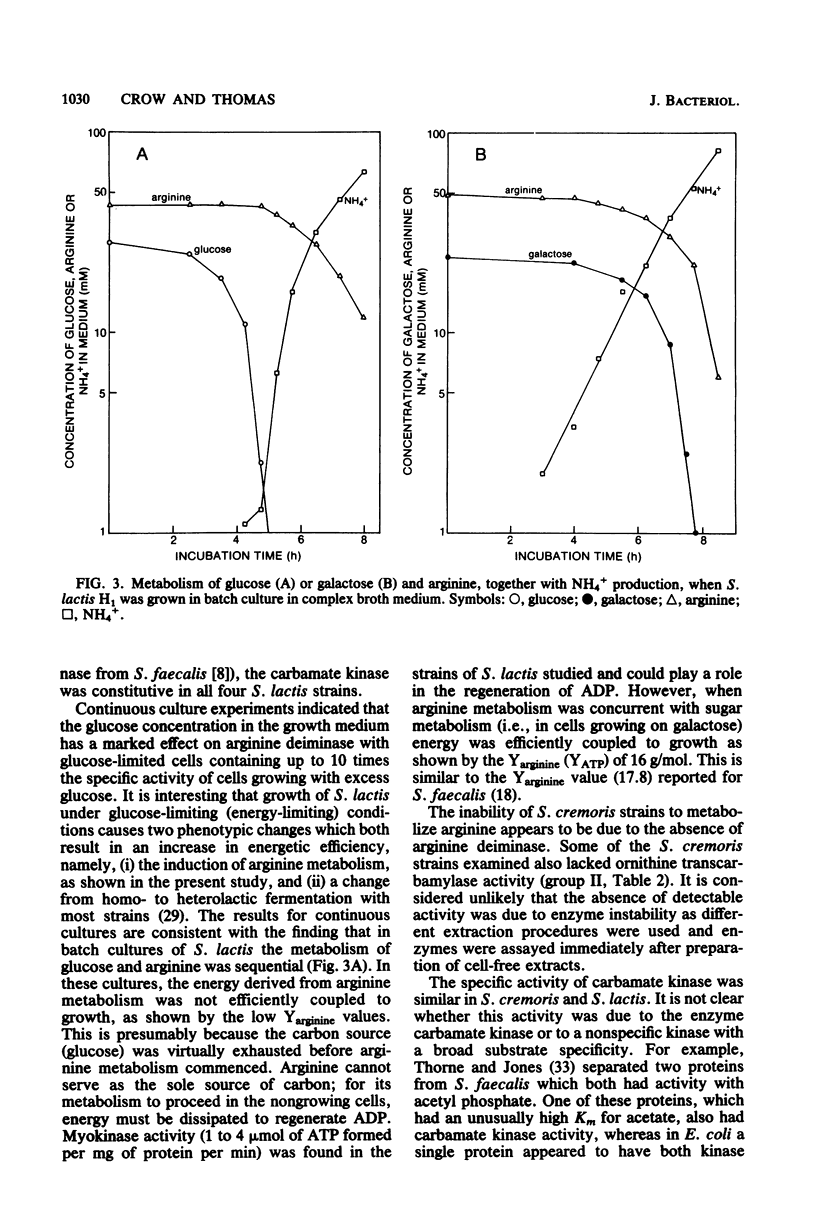
Streptococcus lactis metabolizes arginine via the arginine deiminase pathway producing ornithine, ammonia, carbon dioxide, and ATP. In the four strains of S. lactis examined, the specific activities of arginine deiminase and ornithine transcarbamylase were 5- to 10-fold higher in galactose-grown cells compared with glucose- or lactose-grown cells. The addition of arginine increased the specific activities of these two enzymes with all growth sugars. The specific activity of the third enzyme involved in arginine metabolism (carbamate kinase) was not altered by the composition of the growth medium. In continuous cultures arginine deiminase was not induced, and arginine was not metabolized, until glucose limitation occurred. In batch cultures the metabolism of glucose and arginine was sequential, whereas galactose and arginine were metabolized concurrently, and the energy derived from arginine metabolism was efficiently coupled to growth. No arginine deiminase activity was detected in the nine Streptococcus cremoris strains examined, thus accounting for their inability to metabolize arginine. All nine strains of S. cremoris had specific activities of carbamate kinase similar to those found in S. lactis, but only five S. cremoris strains had ornithine transcarbamylase activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Abrams A., Smith J. B., Baron C. Carbodiimide-resistant membrane adenosine triphosphatase in mutants of Streptococcus faecalis. I. Studies of the mechanism of resistance. J Biol Chem. 1972 Mar 10;247(5):1484–1488. [PubMed] [Google Scholar]
- Editorial Note. Bacteriol Rev. 1937 Dec;1(1):1.b1–1.b1. doi: 10.1128/br.1.1.1.b1-1.b1.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hills G. M. Ammonia production by pathogenic bacteria. Biochem J. 1940 Jul;34(7):1057–1069. doi: 10.1042/bj0341057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALMAN S. M., DUFFIELD P. H. PURIFICATION AND PROPERTIES OF CARBAMATE KINASE FROM STREPTOCOCCUS FAECALIS. Biochim Biophys Acta. 1964 Dec 23;92:498–512. doi: 10.1016/0926-6569(64)90010-0. [DOI] [PubMed] [Google Scholar]
- KORZENOVSKY M., WERKMAN C. H. Conversion of citrulline to ornithine by cell-free extracts of Streptococcus lactis. Arch Biochem Biophys. 1953 Sep;46(1):174–185. doi: 10.1016/0003-9861(53)90180-5. [DOI] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. A kinetic study of the mechanism of crystalline carbamate kinase. J Biol Chem. 1966 Sep 25;241(18):4197–4208. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. 3. Effects of chemical modifications of specific residues on ligand binding and enzymatic activity. J Biol Chem. 1972 Mar 25;247(6):1669–1682. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. II. Multiple binding sites for carbamyl-P and L-norvaline, correlation with steady state kinetics. J Biol Chem. 1972 Mar 25;247(6):1654–1668. [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- Moustafa H. H., Collins E. B. Molar growth yields of certain lactic acid bacteria as influenced by autolysis. J Bacteriol. 1968 Jul;96(1):117–125. doi: 10.1128/jb.96.1.117-125.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven C. F., Smiley K. L., Sherman J. M. The Hydrolysis of Arginine by Streptococci. J Bacteriol. 1942 Jun;43(6):651–660. doi: 10.1128/jb.43.6.651-660.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRACK B., SULLIVAN L., RATNER S. Behavior of purified arginine desiminase from S. faecalis. Arch Biochem Biophys. 1957 Jul;69:186–197. doi: 10.1016/0003-9861(57)90485-x. [DOI] [PubMed] [Google Scholar]
- Pandey V. N., Pradhan D. S. Reverse and forward reactions of carbamyl phosphokinase from Streptococcus faecalis R. Participation of nucleotides and reaction mechanisms. Biochim Biophys Acta. 1981 Aug 13;660(2):284–292. doi: 10.1016/0005-2744(81)90172-8. [DOI] [PubMed] [Google Scholar]
- RAVEL J. M., GRONA M. L., HUMPHREYS J. S., SHIVE W. Properties and biotin content of purified preparations of the ornithinecitrulline enzyme of Streptococcus lactis. J Biol Chem. 1959 Jun;234(6):1452–1455. [PubMed] [Google Scholar]
- RAVEL J. M., HUMPHREYS J. S., SHIVE W. A study of carbamyl phosphate synthesis in Streptococcus lactis. Arch Biochem Biophys. 1961 Mar;92:525–531. doi: 10.1016/0003-9861(61)90393-9. [DOI] [PubMed] [Google Scholar]
- Reddy M. S., Vedamuthu E. R., Washam C. J., Reinbold G. W. Differential agar medium for separating Streptococcus lactis and Streptococcus cremoris. Appl Microbiol. 1969 Nov;18(5):755–759. doi: 10.1128/am.18.5.755-759.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE K. J., JONES M. E. CARBAMYL AND ACETYL PHOSPHOKINASE ACTIVITIES OF STREPTOCOCCUS FAECALIS AND ESCHERICHIA COLI. J Biol Chem. 1963 Sep;238:2992–2998. [PubMed] [Google Scholar]
- Thomas T. D., Jarvis B. D., Skipper N. A. Localization of proteinase(s) near the cell surface of Streptococcus lactis. J Bacteriol. 1974 May;118(2):329–333. doi: 10.1128/jb.118.2.329-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Turner K. W., Crow V. L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980 Nov;144(2):672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


