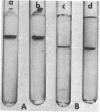
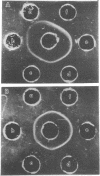
Abstract
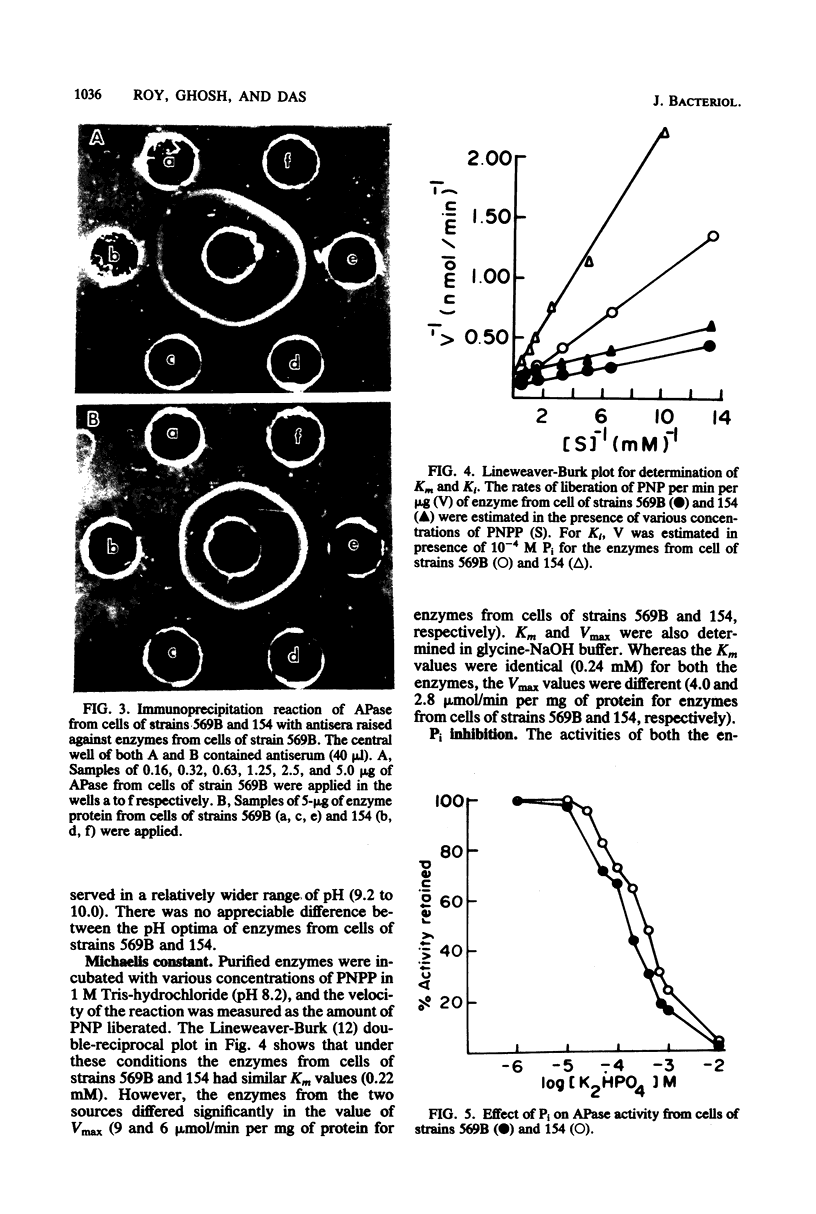
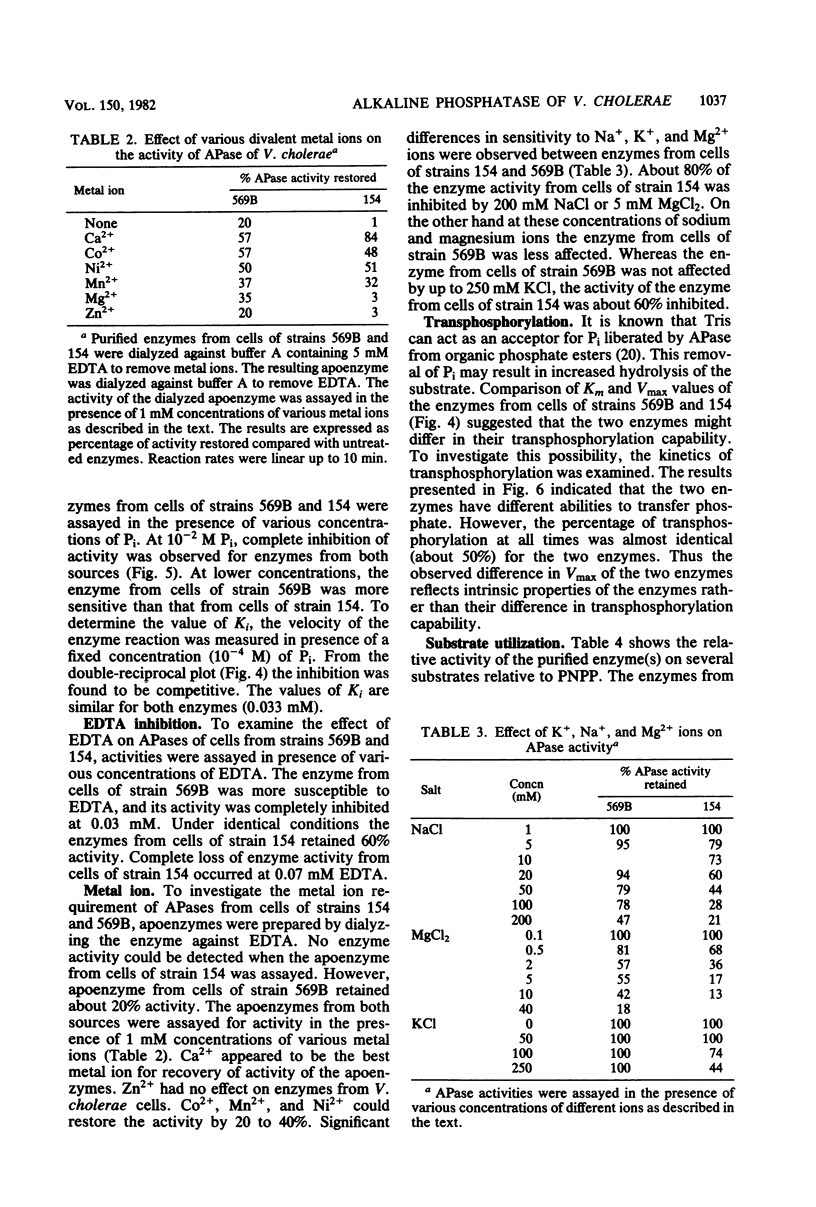
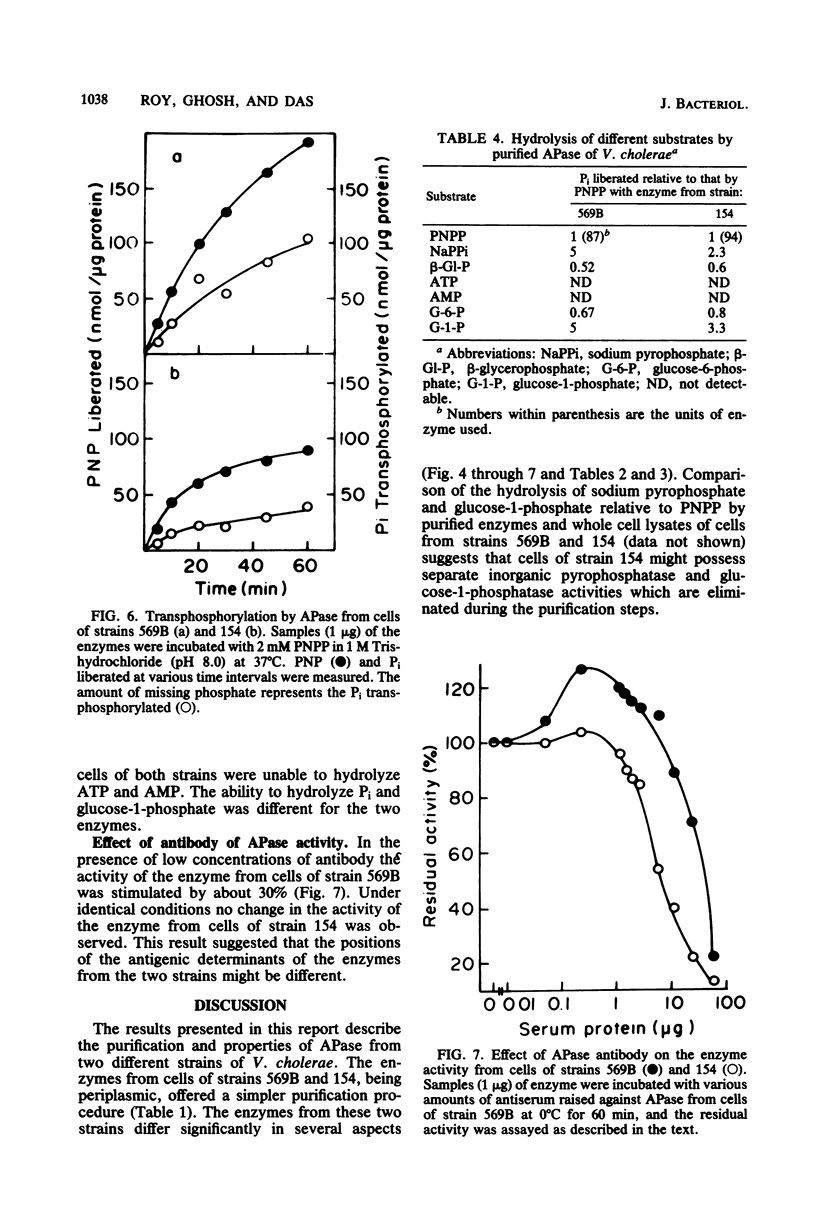
Alkaline phosphatase has been purified to homogeneity from two strains of Vibrio cholerae. The enzymes from both strains are single polypeptides of molecular weight 60,000. Both of the enzymes have pH optima around 8.0 and can act on a variety of organic phosphate esters, glucose-1-phosphate being the best substrate. The enzymes are unable to hydrolyze ATP and AMP. Although they have identical Km values, the two enzymes differ significantly in Vmax with p-nitrophenyl phosphate as substrate. The enzymes from the two strains also differ in their sensitivity to EDTA, Pi, and metal ions and activities of the apoenzymes. Ca2+ reactivated the apoenzymes most.
Full text
PDF
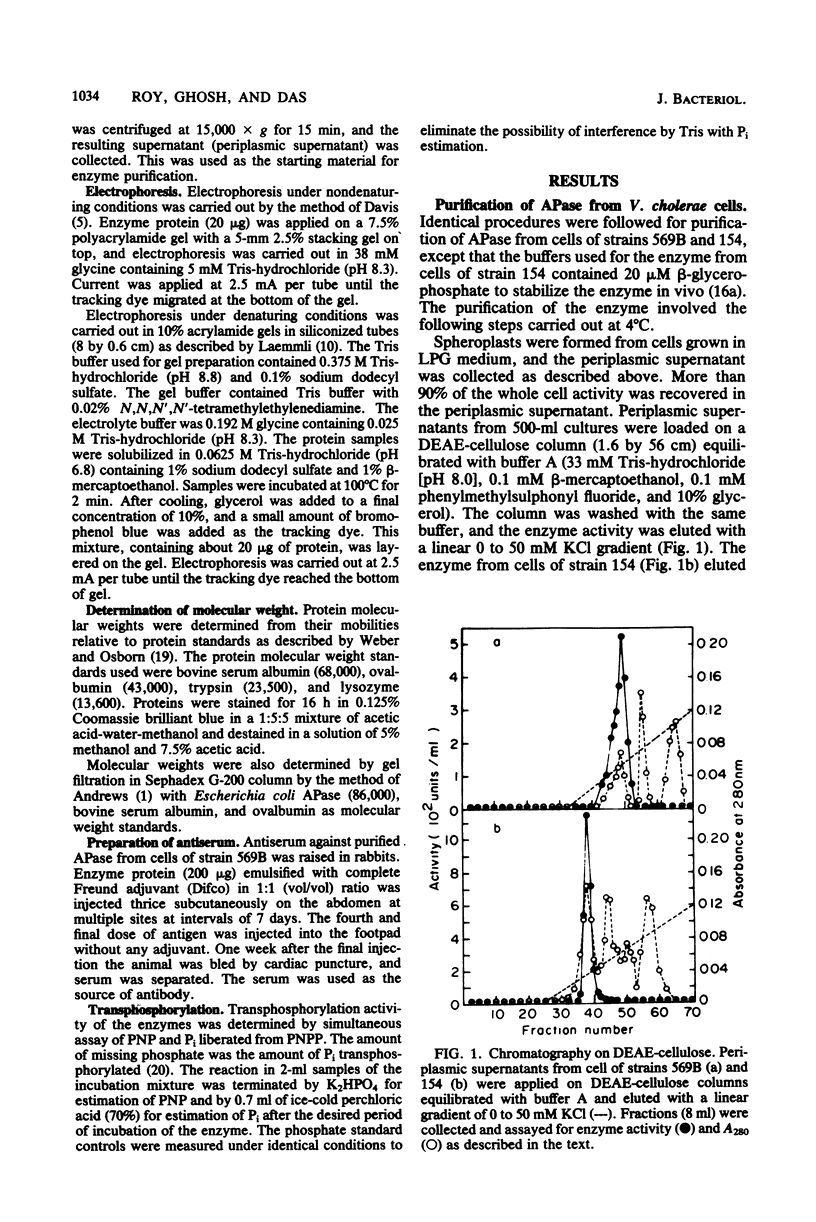
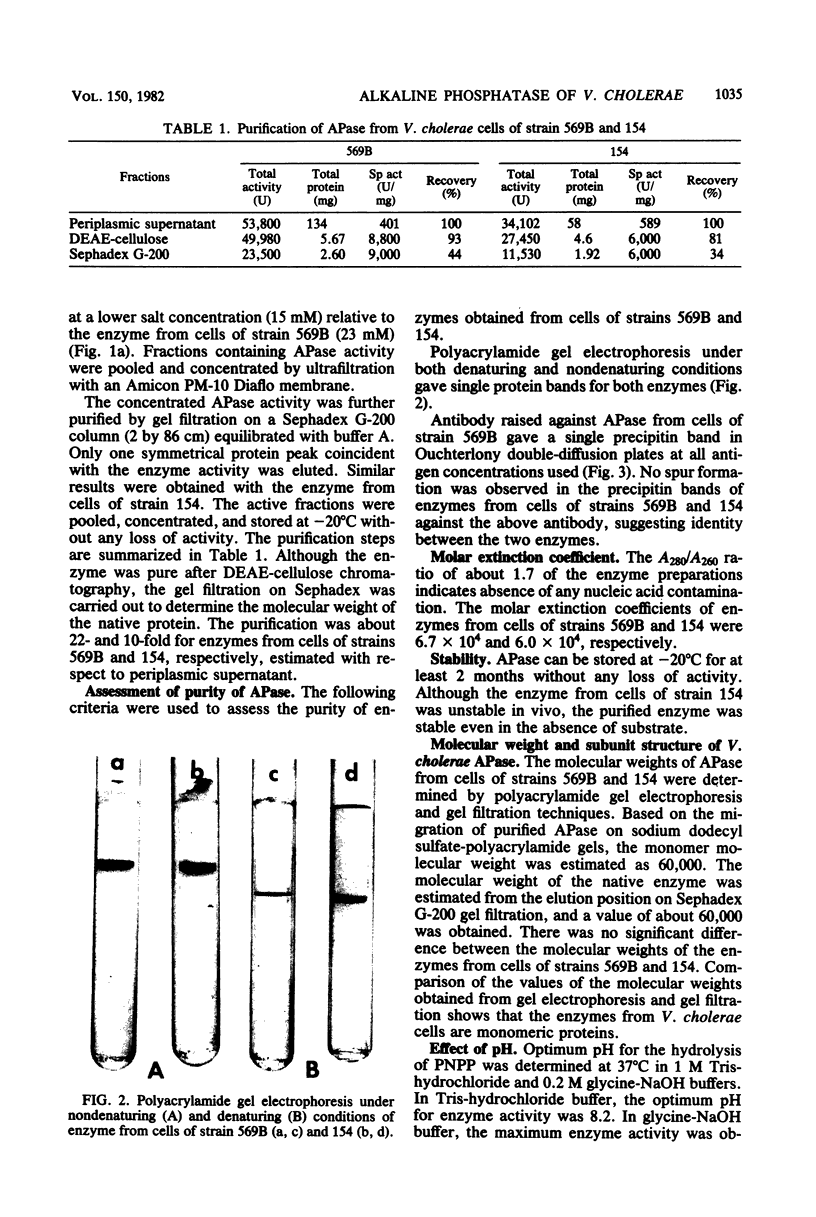

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosron W. F., Kennedy F. S., Vallee B. L. Zinc and magnesium content of alkaline phosphatase from Escherichia coli. Biochemistry. 1975 May 20;14(10):2275–2282. doi: 10.1021/bi00681a036. [DOI] [PubMed] [Google Scholar]
- Das G., Sil K., Das J. Repair of ultraviolet-light-induced DNA damage in vibrio cholerae. Biochim Biophys Acta. 1981 Oct 27;655(3):413–420. doi: 10.1016/0005-2787(81)90053-8. [DOI] [PubMed] [Google Scholar]
- Ghosh R., Ghosh A., Ghosh B. K. Properties of the membrane-bound alkaline phosphatase from glucose- and lactate-grown cells of Bacillus subtilis SB 15. J Biol Chem. 1977 Oct 10;252(19):6813–6822. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Petitclerc C., Chappelet D., Lazdunski C. Flip-flop mechanisms in enzymology. A model: the alkaline phosphatase of Escherichia coli. Eur J Biochem. 1971 May 11;20(1):124–139. doi: 10.1111/j.1432-1033.1971.tb01370.x. [DOI] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- ROTHMAN F., BYRNE R. Fingerprint analysis of alkaline phosphatase of Escherichia coli K12. J Mol Biol. 1963 Apr;6:330–340. doi: 10.1016/s0022-2836(63)80092-3. [DOI] [PubMed] [Google Scholar]
- Roy N. K., Ghosh R. K., Das J. Repression of the alkaline phosphatase of Vibrio cholerae. J Gen Microbiol. 1982 Feb;128(2):349–353. doi: 10.1099/00221287-128-2-349. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Secretion of alkaline phosphatase subunits by spheroplasts of Escherichia coli. J Bacteriol. 1968 Sep;96(3):727–733. doi: 10.1128/jb.96.3.727-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn M. J. A simple test for inactivation of an enzyme during assay. Biochim Biophys Acta. 1965 Jul 29;105(1):193–195. doi: 10.1016/s0926-6593(65)80190-4. [DOI] [PubMed] [Google Scholar]
- WILSON I. B., DAYAN J., CYR K. SOME PROPERTIES OF ALKALINE PHOSPHATASE FROM ESCHERICHIA COLI. TRANSPHOSPHORYLATION. J Biol Chem. 1964 Dec;239:4182–4185. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]