Abstract
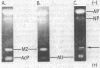
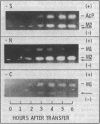
Three electrophoretically distinct acid proteases appear in culture filtrates of Neurospora crassa. Like the previously investigated alkaline and neutral proteases, these enzymes require induction by an exogenous protein. But in contrast to alkaline and neutral proteases, which are synthesized and secreted in response to limitation of any one of three nutrilites (carbon, nitrogen or sulfur), extracellular elaboration of the acidic proteases is more specifically a function of the missing nutrilite. AcP, a pepstatin-inhibitable enzyme similar to other fungal carboxyl proteases, was secreted in large amounts when protein was the sole source of sulfur. Only trace amounts were secreted when nitrogen was the limiting nutrilite, and it was undetectable under carbon limitation. M-1, a chelator-sensitive protease, was secreted when nitrogen or carbon was limiting. M-2, also chelator sensitive, was present only when nitrogen or sulfur was limiting. The evidence presented suggests that the differential regulation of the acidic proteases with respect to nutrilite deprivation may not occur at the level of transcription. AcP and M-2 were partially purified from nitrogen-derepressed cultures by ultrafiltration, cation-exchange chromatography, and gel filtration. AcP has a molecular weight of 66,000, is stable from pH 3.0 to 6.0, and is optimally active toward bovine serum albumin at pH 4.0. M-2 has a molecular weight of 18,000, is stable from pH 1.6 to 5.5, and has optimal activity at pH 4.5.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen B. L., Drucker H. Regulation of exocellular protease in Neurospora crassa: induction and repression under conditions of nitrogen starvation. Arch Biochem Biophys. 1977 Aug;182(2):601–613. doi: 10.1016/0003-9861(77)90541-0. [DOI] [PubMed] [Google Scholar]
- Cohen B. L., Morris J. E., Drucker H. Regulation of two extracellular proteases of Neurospora crassa by induction and by carbon-nitrogen and sulfur-metabolite repression. Arch Biochem Biophys. 1975 Jul;169(1):324–330. doi: 10.1016/0003-9861(75)90347-1. [DOI] [PubMed] [Google Scholar]
- Cohen B. L. The neutral and alkaline proteases of Aspergillus nidulans. J Gen Microbiol. 1973 Aug;77(2):521–528. doi: 10.1099/00221287-77-2-521. [DOI] [PubMed] [Google Scholar]
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: induction and repression of enzyme synthesis. J Bacteriol. 1972 Jun;110(3):1041–1049. doi: 10.1128/jb.110.3.1041-1049.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: metabolic requirements of the process. J Bacteriol. 1975 Jun;122(3):1117–1125. doi: 10.1128/jb.122.3.1117-1125.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: role of Neurospora proteases in induction. J Bacteriol. 1973 Nov;116(2):593–599. doi: 10.1128/jb.116.2.593-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A., Marzluf G. A. Regulation of a sulfur-controlled protease in Neurospora crassa. J Bacteriol. 1973 Nov;116(2):785–789. doi: 10.1128/jb.116.2.785-789.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Fecycz I. T., Stemke G. W., Campbell J. N. Demonstration of a cell-associated, inactive precursor of an exocellular protease produced by Pseudomonas aeruginosa. Can J Microbiol. 1980 Jan;26(1):87–93. doi: 10.1139/m80-013. [DOI] [PubMed] [Google Scholar]
- Lindberg R. A., Eirich L. D., Price J. S., Wolfinbarger L., Jr, Drucker H. Alkaline protease from Neurospora crassa. Purification and partial characterization. J Biol Chem. 1981 Jan 25;256(2):811–814. [PubMed] [Google Scholar]
- Marshall W. E., Manion R., Porath J. A non-discriminating proteinase of Penicillium notatum. Partial purification and specificity. Biochim Biophys Acta. 1968 Feb 5;151(2):414–420. doi: 10.1016/0005-2744(68)90109-5. [DOI] [PubMed] [Google Scholar]
- Schwabe C. A fluorescent assay for proteolytic enzymes. Anal Biochem. 1973 Jun;53(2):484–490. doi: 10.1016/0003-2697(73)90098-5. [DOI] [PubMed] [Google Scholar]
- Siepen D., Yu P. H., Kula M. R. Proteolytic enzymes of Neurospora crassa. Purification and some properties of five intracellular proteinases. Eur J Biochem. 1975 Aug 1;56(1):271–281. doi: 10.1111/j.1432-1033.1975.tb02230.x. [DOI] [PubMed] [Google Scholar]
- Tsujita Y., Endo A. Purification and characterization of the two molecular forms of Aspergillus oryzae acid protease. Biochim Biophys Acta. 1976 Aug 12;445(1):194–204. doi: 10.1016/0005-2744(76)90172-8. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Matchett W. H. Alteration of tryptophan-mediated regulation in Neurospora crassa by indoleglycerol phosphate. J Bacteriol. 1968 May;95(5):1608–1614. doi: 10.1128/jb.95.5.1608-1614.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]