Abstract
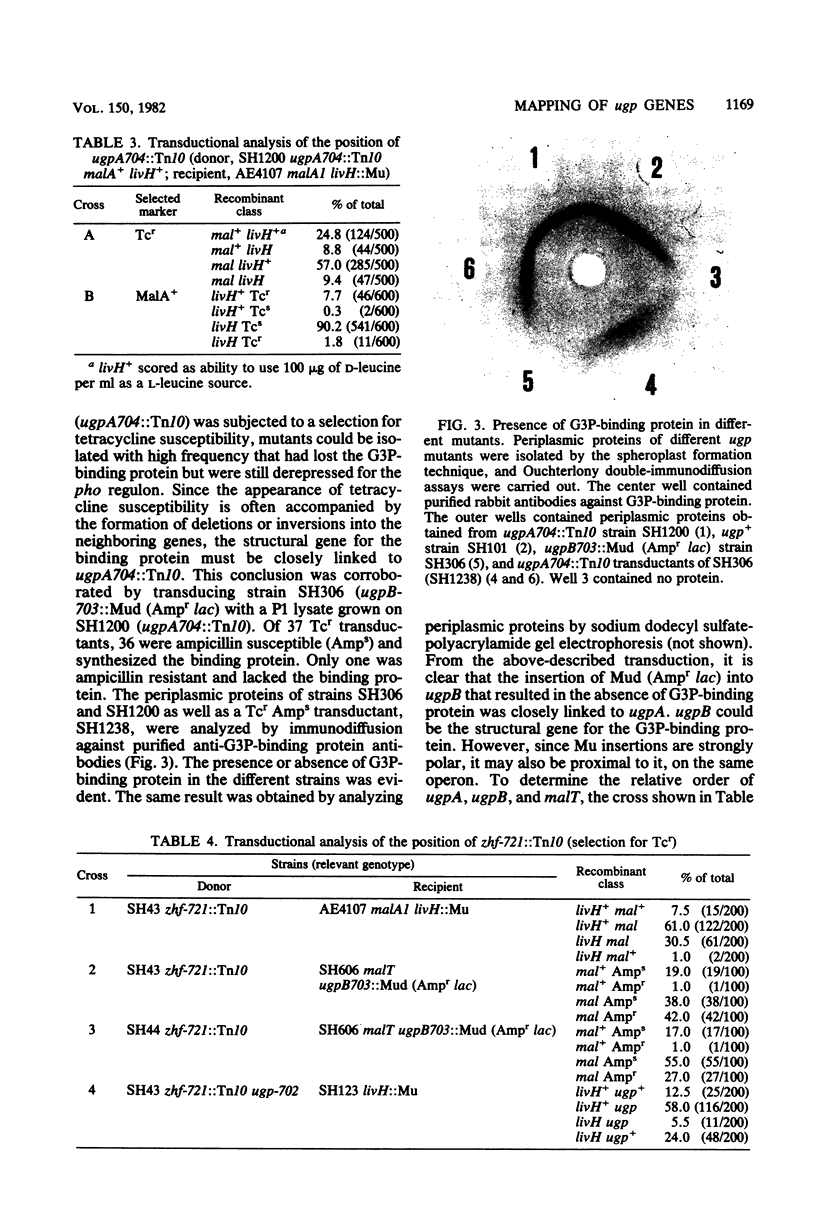
Two genes, ugpA and ugpB, coding for a binding protein-dependent sn-glycerol-3-phosphate transport system, were mapped at 75.3 min on the Escherichia coli chromosome. A Tn10 insertion in ugpA resulted in loss of transport activity but still allowed the synthesis of the sn-glycerol-3-phosphate-binding protein. This Tn10 insertion was found to be linked by P1 transduction to pit, aroB, malA, asd, and livH with 2.5, 2.8, 25, 63.5, and 83% cotransduction frequency. An insertion of Mud (Ampr lac) in ugpB resulted in the loss of the binding protein. ugpB is closely linked to ugpA. It is either the structural gene for the binding protein or located proximal to it. The analysis of the crosses allowed the ordering of the markers in the clockwise direction as follows: aroB, malA, asd, ugpA, ugpB, livH, pit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Oxender D. L. Escherichia coli transport mutants lacking binding protein and other components of the branched-chain amino acid transport systems. J Bacteriol. 1977 Apr;130(1):384–392. doi: 10.1128/jb.130.1.384-392.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono H., Otsuji N. Genetic mapping of regulator gene phoS for alkaline phosphatase in Escherichia coli. J Bacteriol. 1968 Mar;95(3):1182–1183. doi: 10.1128/jb.95.3.1182-1183.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast M., Boos W. Co-regulation in Escherichia coli of a novel transport system for sn-glycerol-3-phosphate and outer membrane protein Ic (e, E) with alkaline phosphatase and phosphate-binding protein. J Bacteriol. 1980 Jul;143(1):142–150. doi: 10.1128/jb.143.1.142-150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Two bacteriophages which utilize a new Escherichia coli major outer membrane protein as part of their receptor. J Bacteriol. 1978 Jul;135(1):164–170. doi: 10.1128/jb.135.1.164-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of sn-glycerol 3-phosphate acyltransferase Km mutants. J Bacteriol. 1974 Oct;120(1):227–233. doi: 10.1128/jb.120.1.227-233.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S K, Adelberg E A. The Occurrence of a Genetic Transposition in a Strain of Escherichia Coli. Genetics. 1962 May;47(5):577–585. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leifer Z., Engel R., Tropp B. E. Transport of 3,4-dihydroxybutyl-1-phosphonate, an analogue of sn-glycerol 3-phosphate. J Bacteriol. 1977 May;130(2):968–971. doi: 10.1128/jb.130.2.968-971.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Zwaig N., Istúriz T., Sánchez R. S. Mutations affecting gluconate metabolism in Escherichia coli. J Bacteriol. 1973 May;114(2):463–468. doi: 10.1128/jb.114.2.463-468.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Sarthy A., Michaelis S., Beckwith J. Use of gene fusions to determine the orientation of gene phoA on the Escherichia coli chromosome. J Bacteriol. 1981 Jan;145(1):293–298. doi: 10.1128/jb.145.1.293-298.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Argast M., Boos W. Characteristics of a binding protein-dependent transport system for sn-glycerol-3-phosphate in Escherichia coli that is part of the pho regulon. J Bacteriol. 1982 Jun;150(3):1154–1163. doi: 10.1128/jb.150.3.1154-1163.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Hartig-Beecken I., Boos W. Periplasmic protein related to the sn-glycerol-3-phosphate transport system of Escherichia coli. J Bacteriol. 1976 May;126(2):951–958. doi: 10.1128/jb.126.2.951-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Bell R. M., Cronan J. E., Jr A mutant of Escherichia coli auxotrophic for organic phosphates: evidence for two defects in inorganic phosphate transport. Mol Gen Genet. 1975 Dec 30;143(1):71–77. doi: 10.1007/BF00269422. [DOI] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]