Abstract
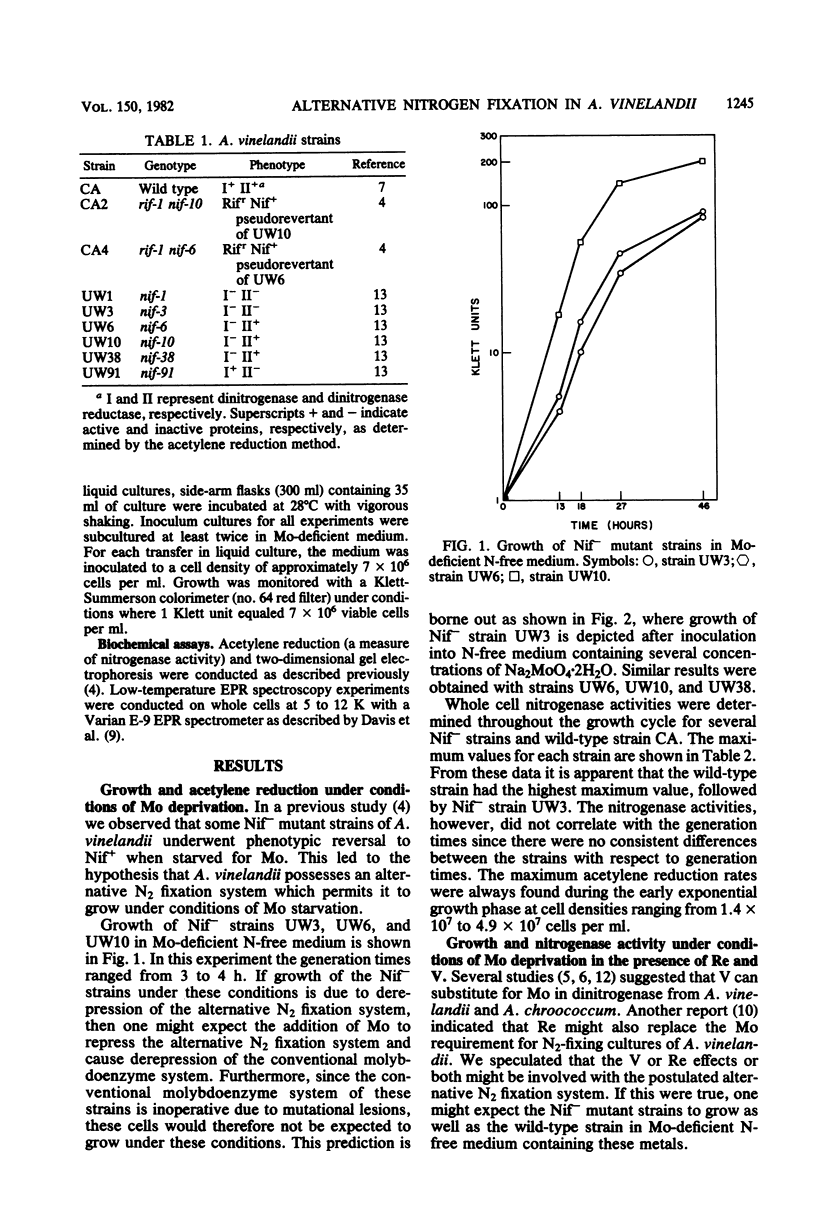
Nitrogenase activities were determined from maximum acetylene reduction rates for mutant strains of Azotobacter vinelandii which are unable to fix N2 in the presence of molybdenum (Nif-) but undergo phenotypic reversal to Nif+ under conditions of Mo deficiency. The system responsible for N2 fixation under these conditions is thought to be an alternative N2 fixation system (Bishop et al., Proc. Natl. Acad. Sci. U.S.A. 77:7342-7346, 1980). Phenotypic reversal of Nif- strains to Nif+ strains was also observed in N-free medium without Mo but with either V or Re. Two protein patterns were found on two-dimensional gels of proteins from the extracts of wild-type cells cultured in N-free medium without Mo and with or without V or Re. The expression of each protein pattern in the wild-type strain of A. vinelandii seemed to depend upon the physiological state of the N2-fixing culture. Electron paramagnetic resonance experiments were conducted on whole cells of A. vinelandii grown under conditions of Mo deprivation in the absence of fixed N. No g = 3.65 signal (an electron paramagnetic resonance signal characteristic of the Mo-containing component of nitrogenase) was detectable in these cells, regardless of whether V or Re was present during growth of these cells, These results are discussed from the perspective that the well-known effect of V on N2 fixation by A. vinelandii may involve an alternative N2 fixation system.
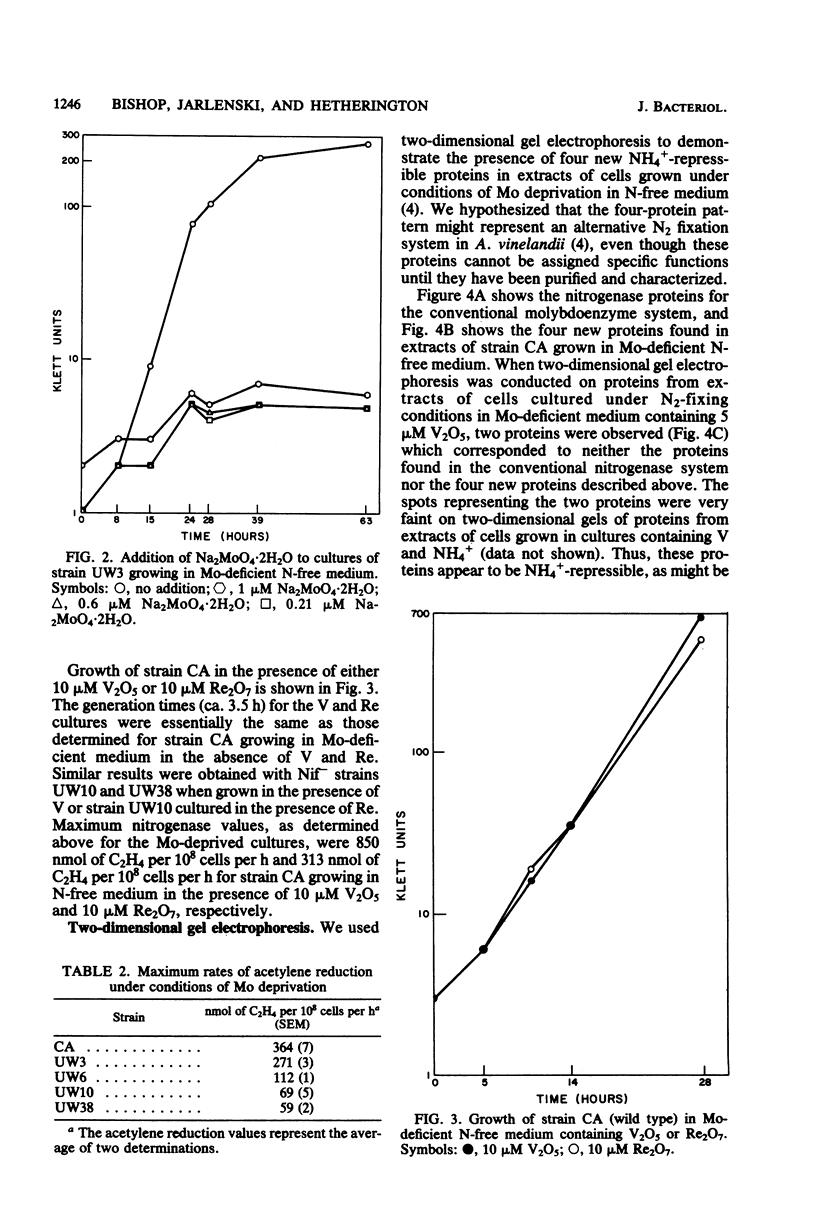
Full text
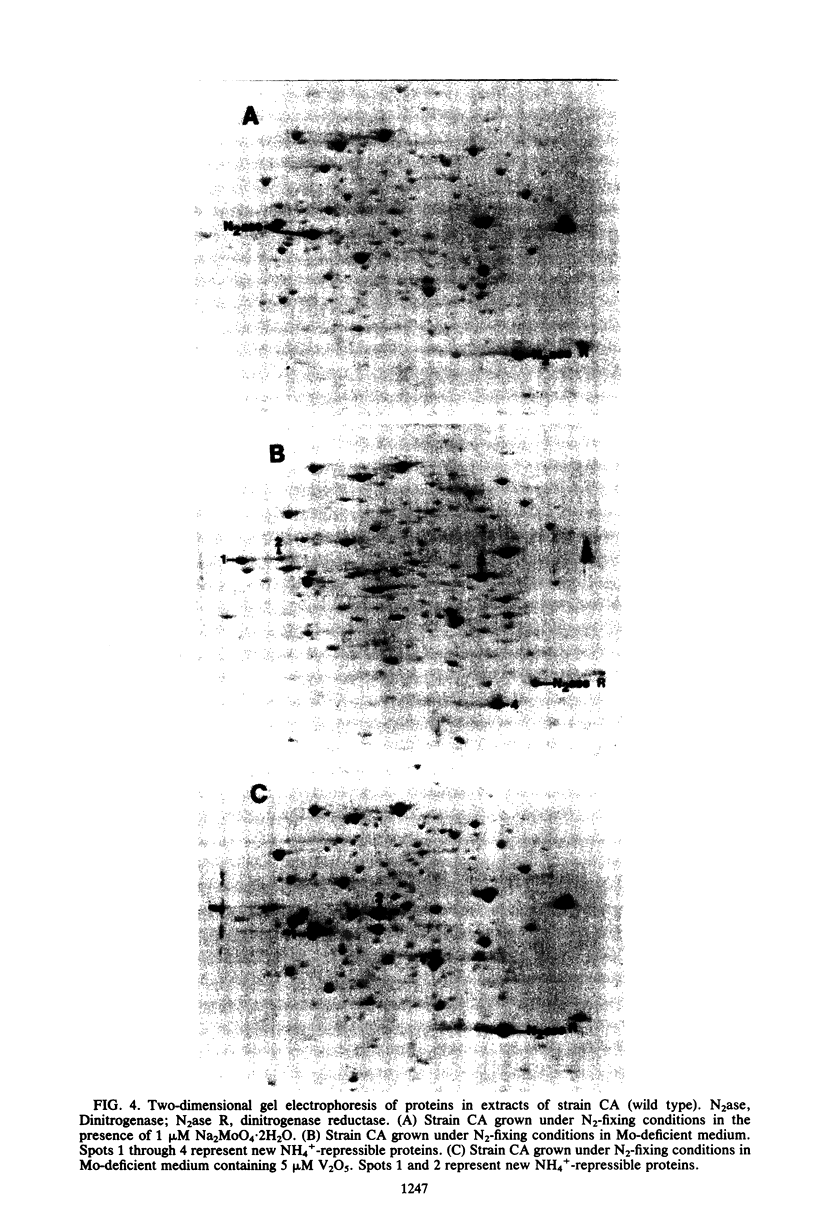
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., McKenna C. E., Lie R. F., Traylor T. G., Kamen M. D. The vanadium effect in nitrogen fixation by azotobacter. Biochim Biophys Acta. 1972 Mar 30;264(1):25–38. doi: 10.1016/0304-4165(72)90113-4. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Brill W. J. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J Bacteriol. 1977 May;130(2):954–956. doi: 10.1128/jb.130.2.954-956.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. C., Fuchsman W. H., Hardy R. W. Nitrogenase from vanadium-grown Azotobacter: isolation, characteristics, and mechanistic implications. Biochem Biophys Res Commun. 1971 Feb 5;42(3):353–358. doi: 10.1016/0006-291x(71)90377-9. [DOI] [PubMed] [Google Scholar]
- DE WITT C. W., ROWE J. A. N,O-Diacetylneuraminic acid and N-acetylneuraminic acid in Escherichia coli. Nature. 1959 Aug 1;184(Suppl 6):381–382. doi: 10.1038/184381b0. [DOI] [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J., Orme-Johnson W. H. Nitrogenase. II. Changes in the EPR signal of component I (iron-molybdenum protein) of Azotobacter vinelandii nitrogenase during repression and derepression. Biochim Biophys Acta. 1972 Feb 28;256(2):512–523. doi: 10.1016/0005-2728(72)90079-5. [DOI] [PubMed] [Google Scholar]
- Fay P., de Vasconcelos L. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. II. The effect of molybdenum and vanadium. Arch Microbiol. 1974;99(3):221–230. doi: 10.1007/BF00696236. [DOI] [PubMed] [Google Scholar]
- McKenna C. E., Benemann J. R., Traylor T. G. A vanadium containing nitrogenase preparation: implications for the role of molybdenum in nitrogen fixation. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1501–1508. doi: 10.1016/0006-291x(70)90557-7. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Swisher R. H., Landt M. L., Reithel F. J. The molecular weight of, and evidence for two types of subunits in, the molybdenum-iron protein of Azotobacter vinelandii nitrogenase. Biochem J. 1977 Jun 1;163(3):427–432. doi: 10.1042/bj1630427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher R. H., Landt M., Reithel F. J. Molecular weights of nitrogenase components from Azotobacter vinelandii. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1476–1482. doi: 10.1016/0006-291x(75)90525-2. [DOI] [PubMed] [Google Scholar]