Abstract
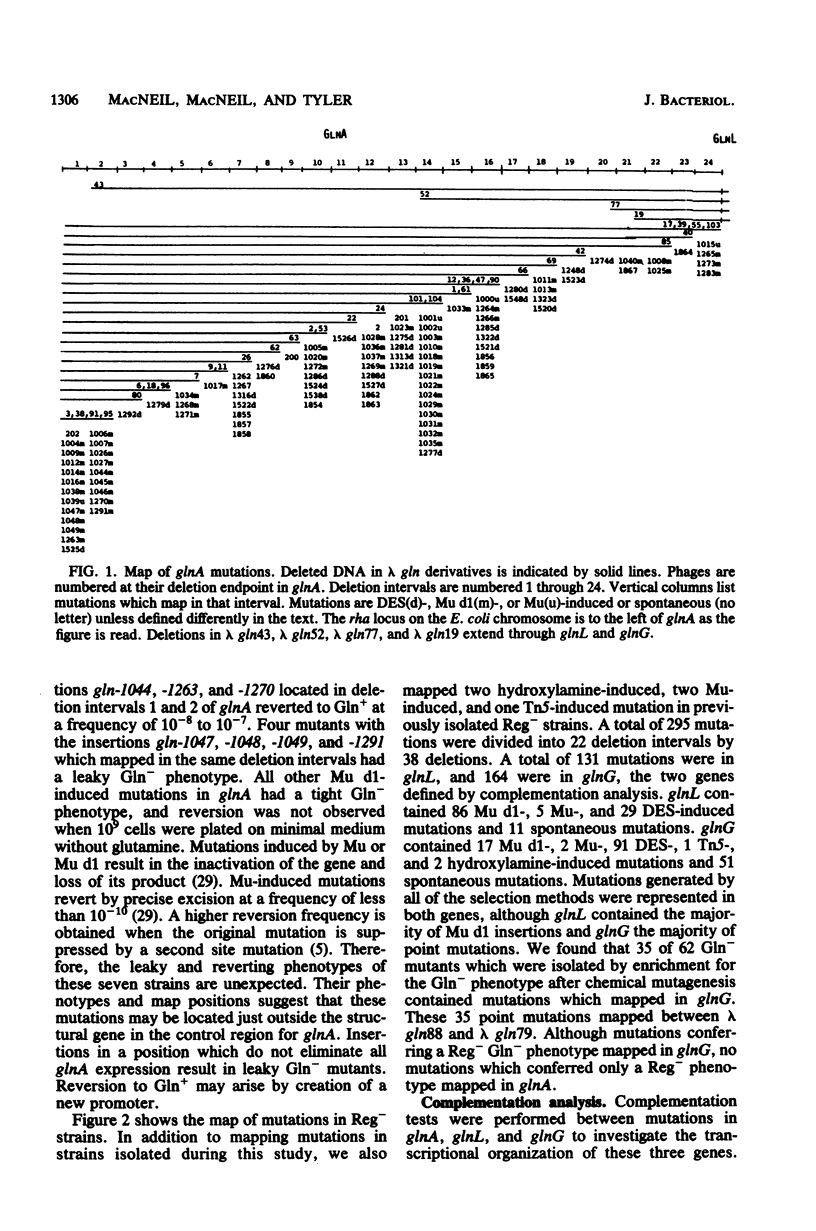
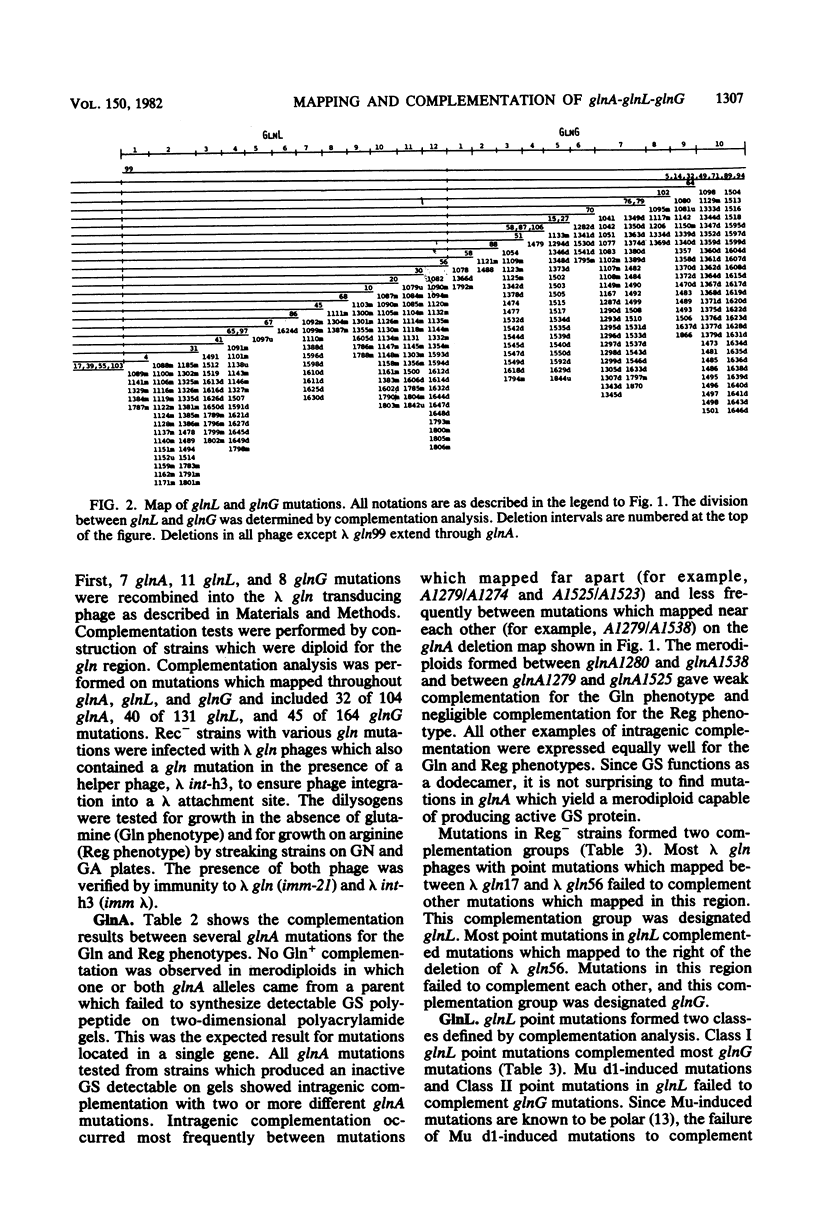
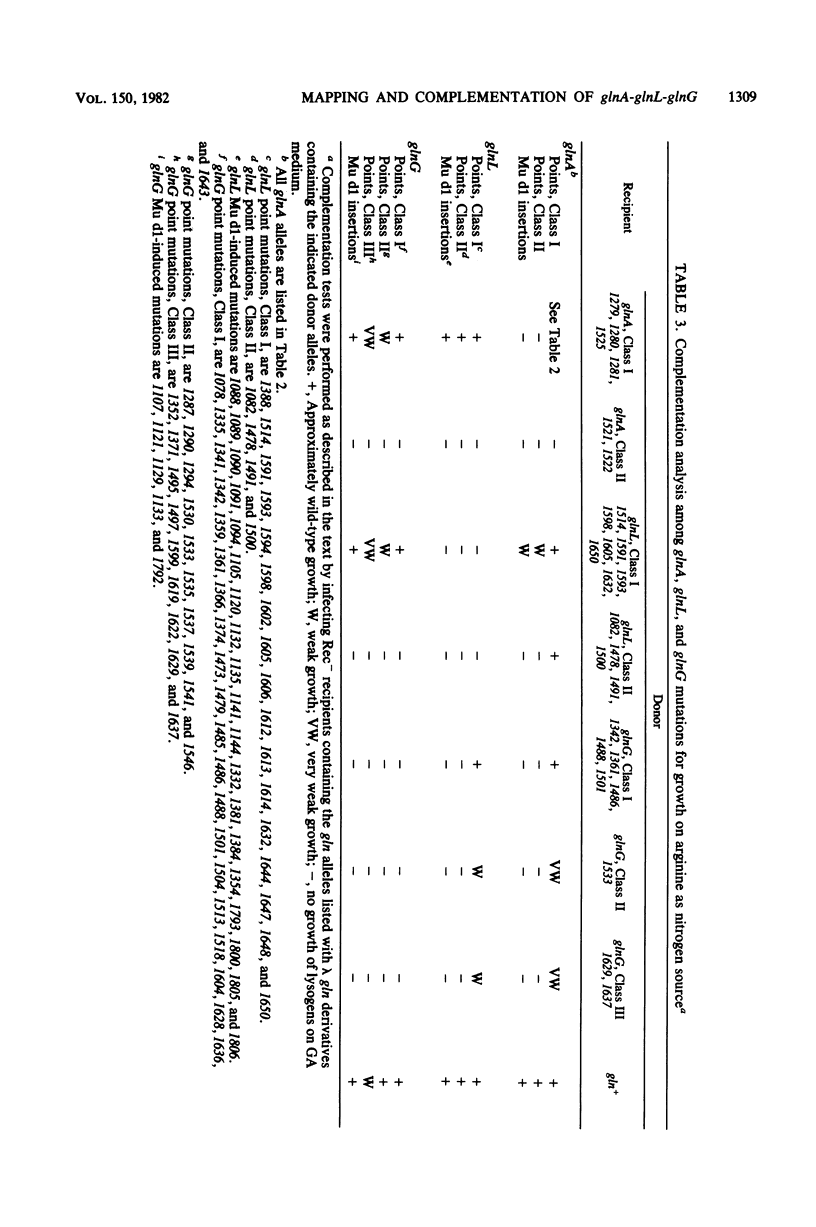
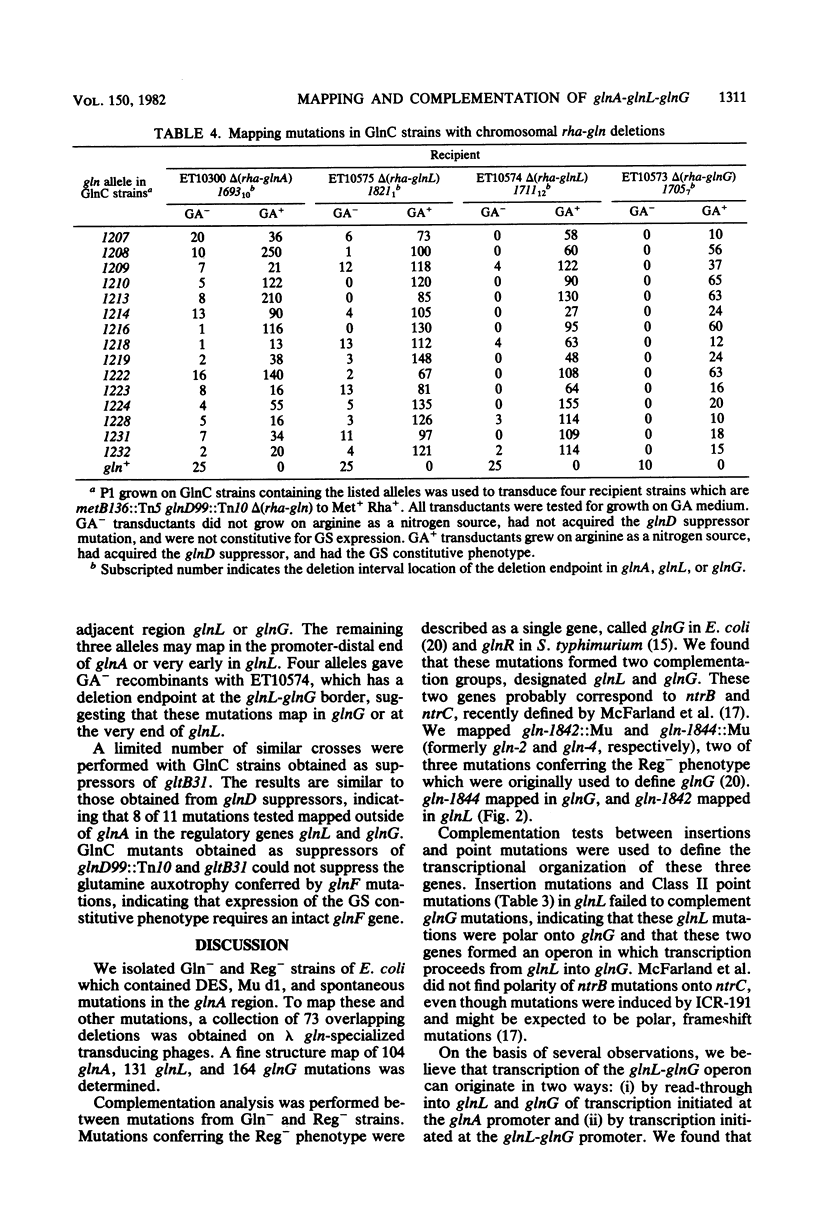
A total of 399 independent mutants of Escherichia coli were obtained which have point and insertion mutations in the glnA region. Mutants isolated included Gln- and Reg- strains (unable to utilize arginine as a nitrogen source). Mutations were mapped with 73 deletion-containing derivatives of a lambda gln phage. Complementation analysis was performed with lambda gln derivatives containing point mutations which conferred a Gln- or Reg- phenotype. Deletion mapping and complementation analysis assigned 104 mutations in 24 deletion intervals to glnA. Mutations in Reg- strains were assigned to two genes, glnL and glnG. glnL contained 131 mutations in 12 deletion intervals, and glnG contained 164 mutations in 10 deletion intervals. The gene order is glnA-glnL-glnG, transcribed from left to right. Polarity of insertion mutations indicates that glnL and glnG form from left to right. Polarity of insertion mutations indicates that glnL and glnG form an operon. Complementation analysis of glnA insertion mutations with glnL and glnG mutations showed polarity of glnA onto most glnL and glnG alleles, suggesting that transcription of glnA may proceed into the glnL-glnG operon. All mutations analyzed in glnA conferred a Gln- phenotype. However, we also found that over half of the Gln- strains isolated ater chemical mutagenesis contained point mutations in glnG. Mutants which synthesized a high level of glutamine synthetase in the presence of ammonia (GlnC phenotype) were selected as revertants of a strain with a Tn10 insertion in glnD and were mapped with chromosomal deletions. Results indicate that mutations in 12 and 15 examined strains clearly map outside of glnA, probably in glnL.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Magasanik B. Autogenous regulation of the synthesis of glutamine synthetase in Klebsiella aerogenes. J Bacteriol. 1977 Oct;132(1):106–112. doi: 10.1128/jb.132.1.106-112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Magasanik B. Regulatory mutations in the Klebsiella aerogenes structural gene for glutamine synthetase. J Bacteriol. 1977 Oct;132(1):100–105. doi: 10.1128/jb.132.1.100-105.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F. R., Levin M. S., Foor F., Tyler B. Regulation of glutamine synthetase formation in Escherichia coli: characterization of mutants lacking the uridylyltransferase. J Bacteriol. 1978 May;134(2):569–577. doi: 10.1128/jb.134.2.569-577.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Genetic analysis of diaminopimelic acid- and lysine-requiring mutants of Escherichia coli. J Bacteriol. 1971 Mar;105(3):844–854. doi: 10.1128/jb.105.3.844-854.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Construction of an Hfr strain useful for transferring recA mutations between Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):529–530. doi: 10.1128/jb.143.1.529-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foor F., Cedergren R. J., Streicher S. L., Rhee S. G., Magasanik B. Glutamine synthetase of Klebsiella aerogenes: properties of glnD mutants lacking uridylyltransferase. J Bacteriol. 1978 May;134(2):562–568. doi: 10.1128/jb.134.2.562-568.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillardin C. M., Magasanik B. Involvement of the product of the glnF gene in the autogenous regulation of glutamine synthetase formation in Klebsiella aerogenes. J Bacteriol. 1978 Mar;133(3):1329–1338. doi: 10.1128/jb.133.3.1329-1338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Bender R. A., Streicher S. L. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974 Jun;118(3):810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. O0 and strong-polar mutations in the gal operon are insertions. Mol Gen Genet. 1968;102(4):353–363. doi: 10.1007/BF00433726. [DOI] [PubMed] [Google Scholar]
- Kelley W. S., Chalmers K., Murray N. E. Isolation and characterization of a lambdapolA transducing phage. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5632–5636. doi: 10.1073/pnas.74.12.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Burton D., Garcia E., McCarter L., McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil D. General method, using Mu-Mud1 dilysogens, to determine the direction of transcription of and generate deletions in the glnA region of Escherichia coli. J Bacteriol. 1981 Apr;146(1):260–268. doi: 10.1128/jb.146.1.260-268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland N., McCarter L., Artz S., Kustu S. Nitrogen regulatory locus "glnR" of enteric bacteria is composed of cistrons ntrB and ntrC: identification of their protein products. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I., Mozola M. A., Friedman D. I. int-h: An int mutation of phage lambda that enhances site-specific recombination. Cell. 1980 Jul;20(3):721–729. doi: 10.1016/0092-8674(80)90318-9. [DOI] [PubMed] [Google Scholar]
- Pahel G., Bloom F. R., Tyler B. Deletion mapping of the polA-metB region of the Escherichia coli chromosome. J Bacteriol. 1979 May;138(2):653–656. doi: 10.1128/jb.138.2.653-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Tyler B. A new glnA-linked regulatory gene for glutamine synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4544–4548. doi: 10.1073/pnas.76.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Zelenetz A. D., Tyler B. M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978 Jan;133(1):139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Rothstein D. M., Pahel G., Tyler B., Magasanik B. Regulation of expression from the glnA promoter of Escherichia coli in the absence of glutamine synthetase. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7372–7376. doi: 10.1073/pnas.77.12.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Halpern Y. S., Magasanik B. Genetic and metabolic control of enzymes responsible for histidine degradation in Salmonella typhimurium. 4-imidazolone-5-propionate amidohydrolase and N-formimino-L-glutamate formiminohydrolase. J Biol Chem. 1971 May 25;246(10):3320–3329. [PubMed] [Google Scholar]
- Streicher S. L., Bender R. A., Magasanik B. Genetic control of glutamine synthetase in Klebiella aerogenes. J Bacteriol. 1975 Jan;121(1):320–331. doi: 10.1128/jb.121.1.320-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S. L., Deleo A. B., Magasanik B. Regulation of enzyme formation in Klebsiella aerogenes by episomal glutamine synthetase of Escherichia coli. J Bacteriol. 1976 Jul;127(1):184–192. doi: 10.1128/jb.127.1.184-192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. M., Goldberg R. B. Transduction of chromosomal genes between enteric bacteria by bacteriophage P1. J Bacteriol. 1976 Mar;125(3):1105–1111. doi: 10.1128/jb.125.3.1105-1111.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weglenski P., Tyler B. Regulation of glnA messinger ribonucleic acid synthesis in Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):880–887. doi: 10.1128/jb.129.2.880-887.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



