Abstract
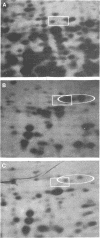
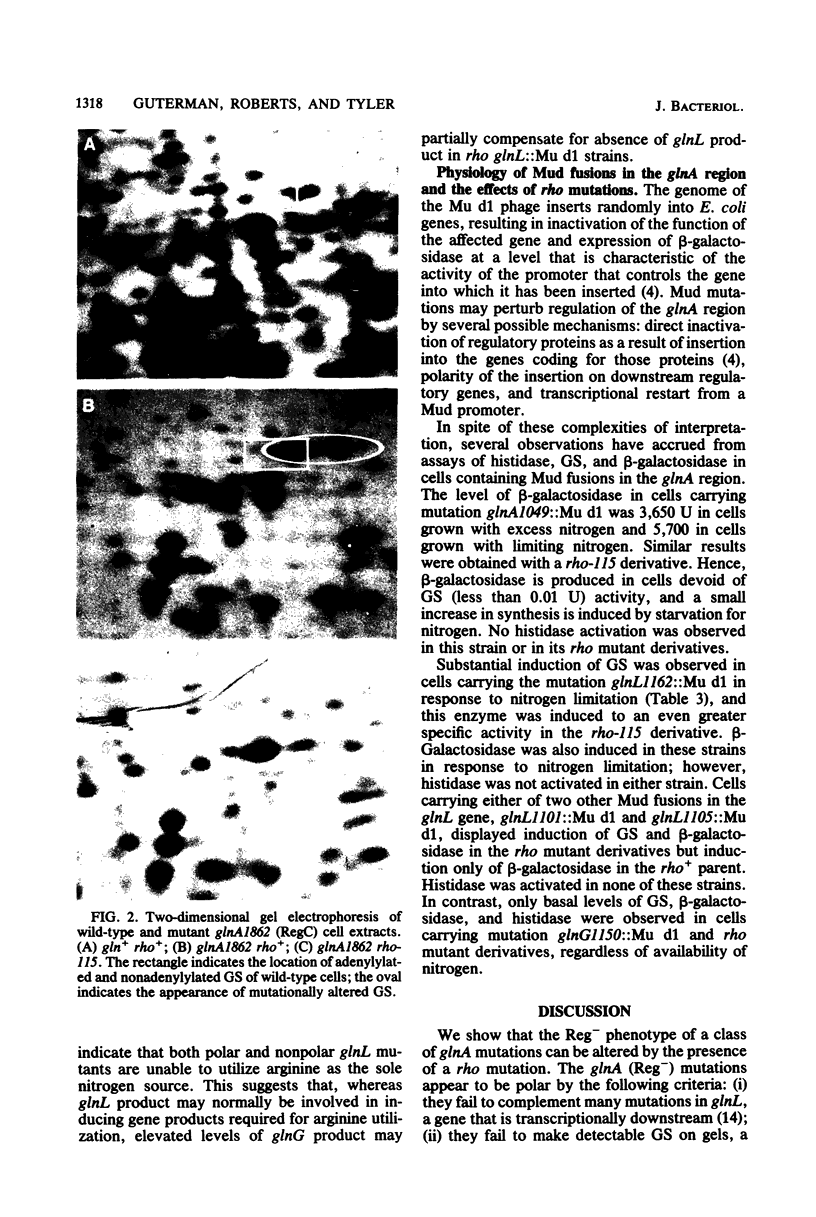
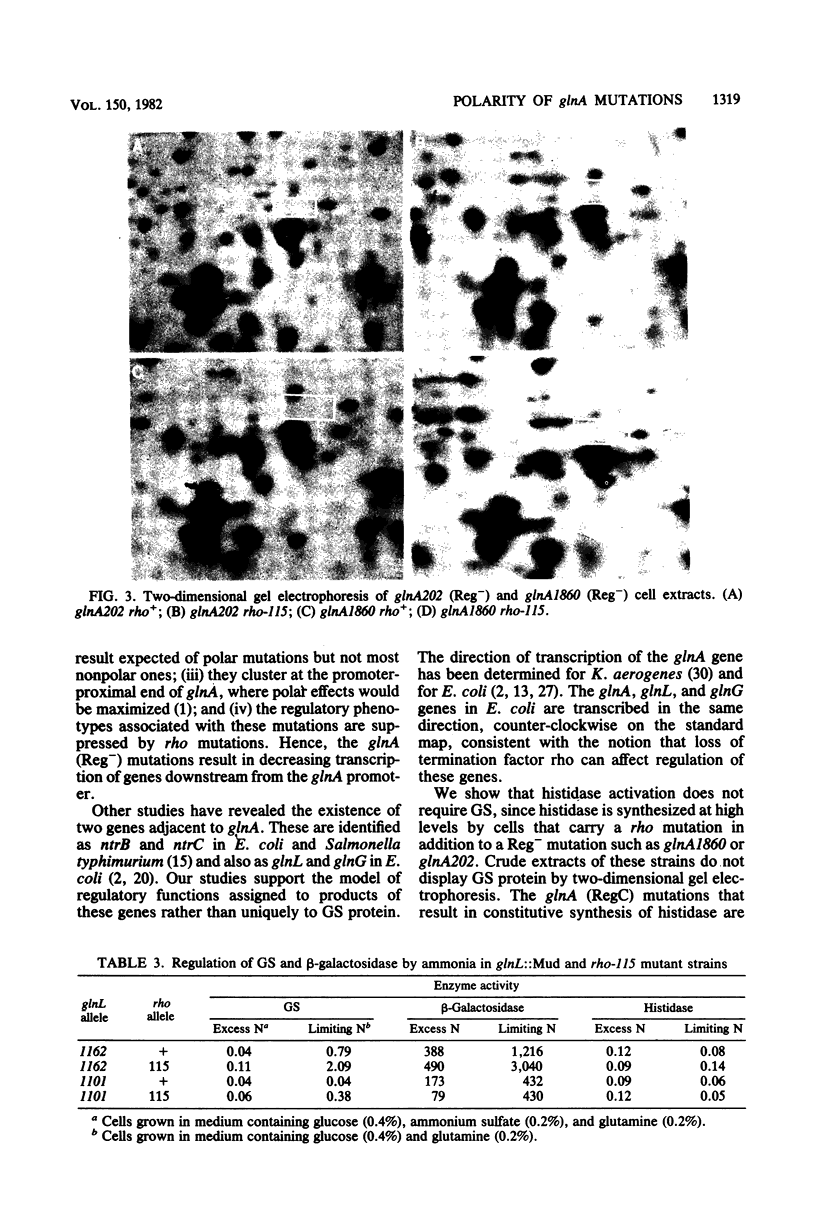
To determine the ability of mutations in glnA, the gene for glutamine synthetase (GS), to regulate nitrogen assimilatory enzymes, we assayed histidase and GS in 34 glnA (Gln−) strains. Twenty-five glnA mutants were RegC, synthesizing high levels of histidase regardless of the availability of nitrogen, and nine were Reg−, synthesizing low levels of histidase in medium containing either limiting or excess ammonia. rho mutations were introduced into strains containing glnA point mutations or insertions in glnA, glnL, glnG, or glnF. The Reg− phenotype of strains with glnA point mutations, but not those with glnA or glnF insertions, was altered by the presence of rho, suggesting that glnA (Reg−) mutations are polar and exert their phenotype by decreasing expression of glnL and glnG. Consistent with this view, no GS protein was detected by two-dimensional gel electrophoresis in glnA (Reg−) rho+ or glnA (Reg−) rho double mutants, whereas GS protein was detected in cells of 10 of 11 glnA (RegC) strains. Since glnA (Reg−) rho double mutants synthesize constitutive levels of histidase, GS protein is not necessary for full expression of histidase. Mu d1 insertions in glnL, but not those in glnG, responded to the presence of a rho allele, presumably owing to elevated transcription into glnG from the Mu d1 prophage. Our results suggest that glnA (Reg−) alleles are polar mutations, and a rho-dependent termination site down-stream is postulated as the basis for the polar phenomenon. The data also indicate that, under some circumstances, a significant portion of glnL and glnG transcription is initiated at the glnA promoter.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Merril C., Adhya S. Interaction of RNA polymerase and rho in transcription termination: coupled ATPase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4828–4832. doi: 10.1073/pnas.75.10.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E., Bancroft S., Rhee S. G., Kustu S. The product of a newly identified gene, gInF, is required for synthesis of glutamine synthetase in Salmonella. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1662–1666. doi: 10.1073/pnas.74.4.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Howitt C. L. Rho and ribosome mutation interaction: lethality of rho-15 in rpsL or rpsE strains, and rho-15 methionine auxotrophy in rps+ strains of Escherichia coli. Genetics. 1979 Oct;93(2):353–360. doi: 10.1093/genetics/93.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Burton D., Garcia E., McCarter L., McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil D. General method, using Mu-Mud1 dilysogens, to determine the direction of transcription of and generate deletions in the glnA region of Escherichia coli. J Bacteriol. 1981 Apr;146(1):260–268. doi: 10.1128/jb.146.1.260-268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Tyler B. Fine-structure deletion map and complementation analysis of the glnA-glnL-glnG region in Escherichia coli. J Bacteriol. 1982 Jun;150(3):1302–1313. doi: 10.1128/jb.150.3.1302-1313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland N., McCarter L., Artz S., Kustu S. Nitrogen regulatory locus "glnR" of enteric bacteria is composed of cistrons ntrB and ntrC: identification of their protein products. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers J. L., Tempest D. W., Brown C. M. 'Glutamine(amide):2-oxoglutarate amino transferase oxido-reductase (NADP); an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol. 1970 Dec;64(2):187–194. doi: 10.1099/00221287-64-2-187. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Guertin M. Amber suA mutations which relieve polarity. J Mol Biol. 1972 Feb 14;63(3):605–608. doi: 10.1016/0022-2836(72)90453-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Tyler B. A new glnA-linked regulatory gene for glutamine synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4544–4548. doi: 10.1073/pnas.76.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Zelenetz A. D., Tyler B. M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978 Jan;133(1):139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Brenchley J. E., Magasanik B. Glutamine synthetase and the regulation of histidase formation in Klebsiella aerogenes. J Biol Chem. 1973 Jun 25;248(12):4334–4344. [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Reyes O., Gottesman M., Adhya S. Suppression of polarity of insertion mutations in the gal operon and N mutations in bacteriophage lambda. J Bacteriol. 1976 Jun;126(3):1108–1112. doi: 10.1128/jb.126.3.1108-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein D. M., Pahel G., Tyler B., Magasanik B. Regulation of expression from the glnA promoter of Escherichia coli in the absence of glutamine synthetase. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7372–7376. doi: 10.1073/pnas.77.12.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S. L., Bender R. A., Magasanik B. Genetic control of glutamine synthetase in Klebiella aerogenes. J Bacteriol. 1975 Jan;121(1):320–331. doi: 10.1128/jb.121.1.320-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S. L., Tyler B. Purification of glutamine synthetase from a variety of bacteria. J Bacteriol. 1980 Apr;142(1):69–78. doi: 10.1128/jb.142.1.69-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weglenski P., Tyler B. Regulation of glnA messinger ribonucleic acid synthesis in Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):880–887. doi: 10.1128/jb.129.2.880-887.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]