Abstract
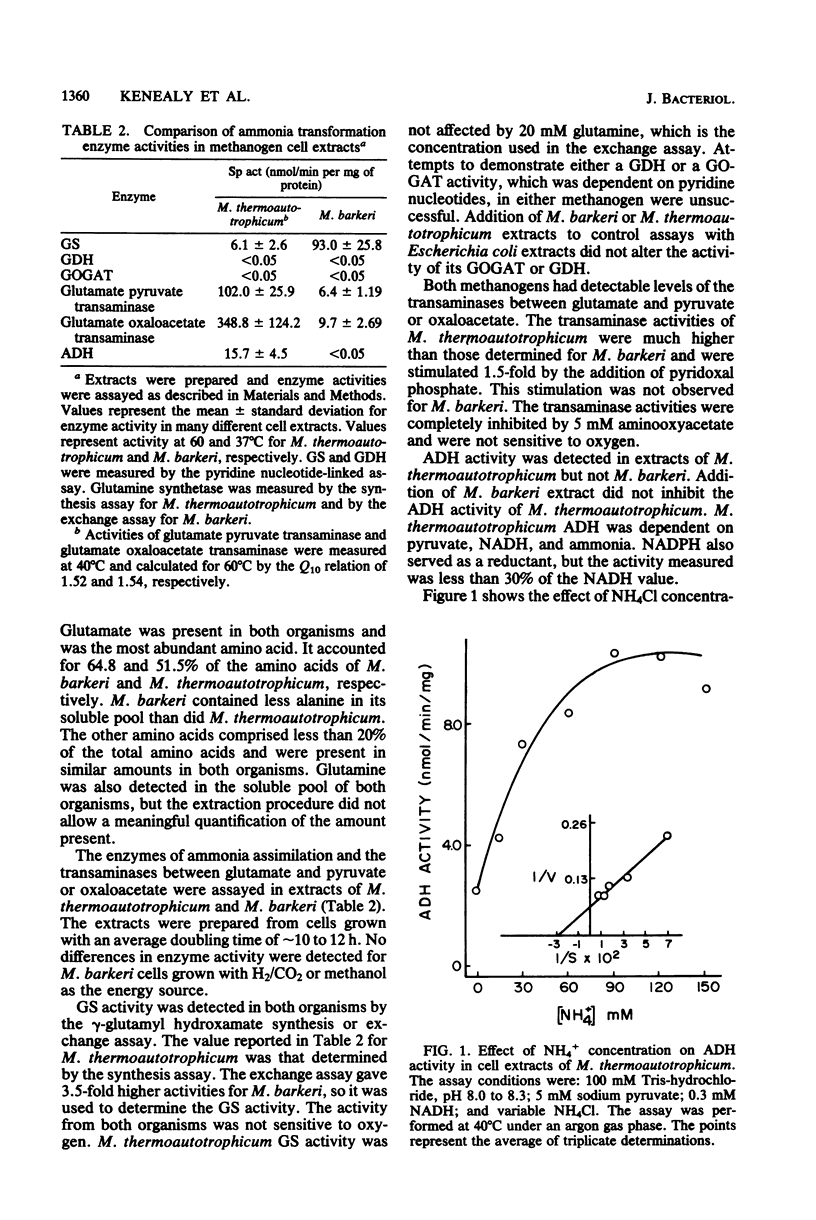
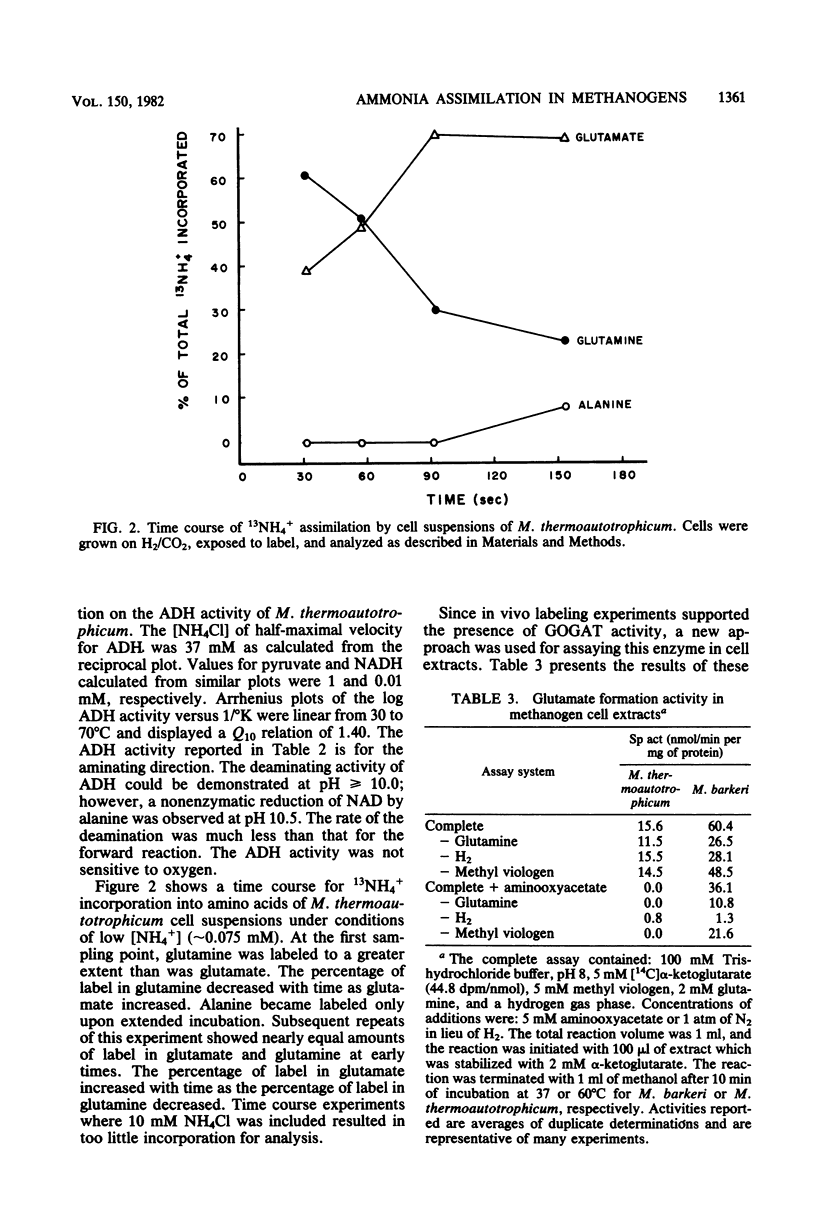
The mechanism of ammonia assimilation in Methanosarcina barkeri and Methanobacterium thermoautotrophicum was documented by analysis of enzyme activities, 13NH3 incorporation studies, and comparison of growth and enzyme activity levels in continuous culture. Glutamate accounted for 65 and 52% of the total amino acids in the soluble pools of M. barkeri and M. thermoautotrophicum. Both organisms contained significant activities of glutamine synthetase, glutamate synthase, glutamate oxaloacetate transaminase, and glutamate pyruvate transaminase. Hydrogen-reduced deazaflavin-factor 420 or flavin mononucleotide but not NAD, NADP, or ferredoxin was used as the electron donor for glutamate synthase in M. barkeri. Glutamate dehydrogenase activity was not detected in either organism, but alanine dehydrogenase activity was present in M. thermoautotrophicum. The in vivo activity of the glutamine synthetase was verified in M. thermoautotrophicum by analysis of 13NH3 incorporation into glutamine, glutamate, and alanine. Alanine dehydrogenase and glutamine synthetase activity varied in response to [NH4+] when M. thermoautotrophicum was cultured in a chemostat with cysteine as the sulfur source. Alanine dehydrogenase activity and growth yield (grams of cells/mole of methane) were highest when the organism was cultured with excess ammonia, whereas growth yield was lower and glutamine synthetase was maximal when ammonia was limiting.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Friedrich C. G. Alanine dehydrogenase of the beta-lactam antibiotic producer Streptomyces clavuligerus. Arch Microbiol. 1980 Mar;125(1-2):137–142. doi: 10.1007/BF00403210. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of 14CO2 and 14CH3OH incorporated into whole cells. J Bacteriol. 1978 Oct;136(1):75–84. doi: 10.1128/jb.136.1.75-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirich L. D., Vogels G. D., Wolfe R. S. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry. 1978 Oct 31;17(22):4583–4593. doi: 10.1021/bi00615a002. [DOI] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E., Thauer R. K. Acetate assimilation and the synthesis of alanine, aspartate and glutamate in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Apr 27;117(1):61–66. doi: 10.1007/BF00689352. [DOI] [PubMed] [Google Scholar]
- Germano G. J., Anderson K. E. Purification and properties of L-alanine dehydrogenase from Desulfovibrio desulfuricans. J Bacteriol. 1968 Jul;96(1):55–60. doi: 10.1128/jb.96.1.55-60.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. S., Stadtman E. R. Regulation of glutamine synthetase. II. Patterns of feedback inhibition in microorganisms. J Bacteriol. 1967 Mar;93(3):1045–1055. doi: 10.1128/jb.93.3.1045-1055.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. C., Gest H. Inorganic nitrogen assimilation by the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1976 Nov;128(2):683–688. doi: 10.1128/jb.128.2.683-688.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy W., Zeikus J. G. Influence of corrinoid antagonists on methanogen metabolism. J Bacteriol. 1981 Apr;146(1):133–140. doi: 10.1128/jb.146.1.133-140.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keradjopoulos D., Wulff K. Thermophilic alanine dehydrogenase from Halobacterium salinarium. Can J Biochem. 1974 Nov;52(11):1033–1037. doi: 10.1139/o74-144. [DOI] [PubMed] [Google Scholar]
- Kim E. K., Fitt P. S. Partial purification and properties of Halobacterium cutirubrum L-alanine dehydrogenase. Biochem J. 1977 Feb 1;161(2):313–320. doi: 10.1042/bj1610313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R., Zeikus J. G. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J Bacteriol. 1980 Nov;144(2):569–578. doi: 10.1128/jb.144.2.569-578.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Glutamate synthase in blue-green algae. Biochem Soc Trans. 1975;3(3):381–384. doi: 10.1042/bst0030381. [DOI] [PubMed] [Google Scholar]
- McCullough H. The determination of ammonia in whole blood by a direct colorimetric method. Clin Chim Acta. 1967 Aug;17(2):297–304. doi: 10.1016/0009-8981(67)90133-7. [DOI] [PubMed] [Google Scholar]
- Meeks J. C., Wolk C. P., Thomas J., Lockau W., Shaffer P. W., Austin S. M., Chien W. S., Galonsky A. The pathways of assimilation of 13NH4+ by the cyanobacterium, Anabaena cylindrica. J Biol Chem. 1977 Nov 10;252(21):7894–7900. [PubMed] [Google Scholar]
- Meers J. L., Tempest D. W., Brown C. M. 'Glutamine(amide):2-oxoglutarate amino transferase oxido-reductase (NADP); an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol. 1970 Dec;64(2):187–194. doi: 10.1099/00221287-64-2-187. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Hespell R. B., Bryant M. P. Ammonia assimilation and glutamate formation in the anaerobe Selenomonas ruminantium. J Bacteriol. 1980 Feb;141(2):593–602. doi: 10.1128/jb.141.2.593-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- Thomas J., Meeks J. C., Wolk C. P., Shaffer P. W., Austin S. M. Formation of glutamine from [13n]ammonia, [13n]dinitrogen, and [14C]glutamate by heterocysts isolated from Anabaena cylindrica. J Bacteriol. 1977 Mar;129(3):1545–1555. doi: 10.1128/jb.129.3.1545-1555.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng S. F., Wolfe R. S., Bryant M. P. Factor 420-dependent pyridine nucleotide-linked hydrogenase system of Methanobacterium ruminantium. J Bacteriol. 1975 Jan;121(1):184–191. doi: 10.1128/jb.121.1.184-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate assimilation pathway of Methanosarcina barkeri. J Bacteriol. 1979 Jan;137(1):332–339. doi: 10.1128/jb.137.1.332-339.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Thomas J., Shaffer P. W., Austin S. M., Galonsky A. Pathway of nitrogen metabolism after fixation of 13N-labeled nitrogen gas by the cyanobacterium, Anabaena cylindrica. J Biol Chem. 1976 Aug 25;251(16):5027–5034. [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



