Abstract
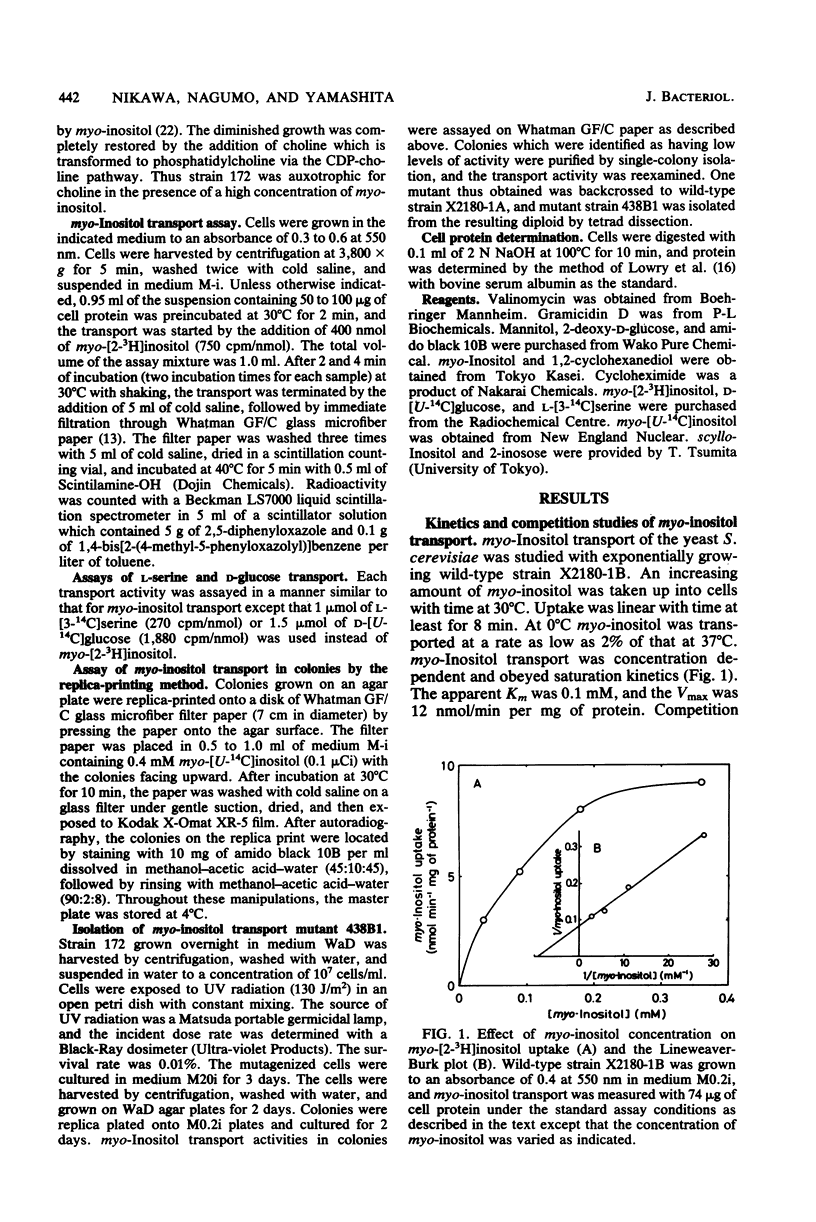
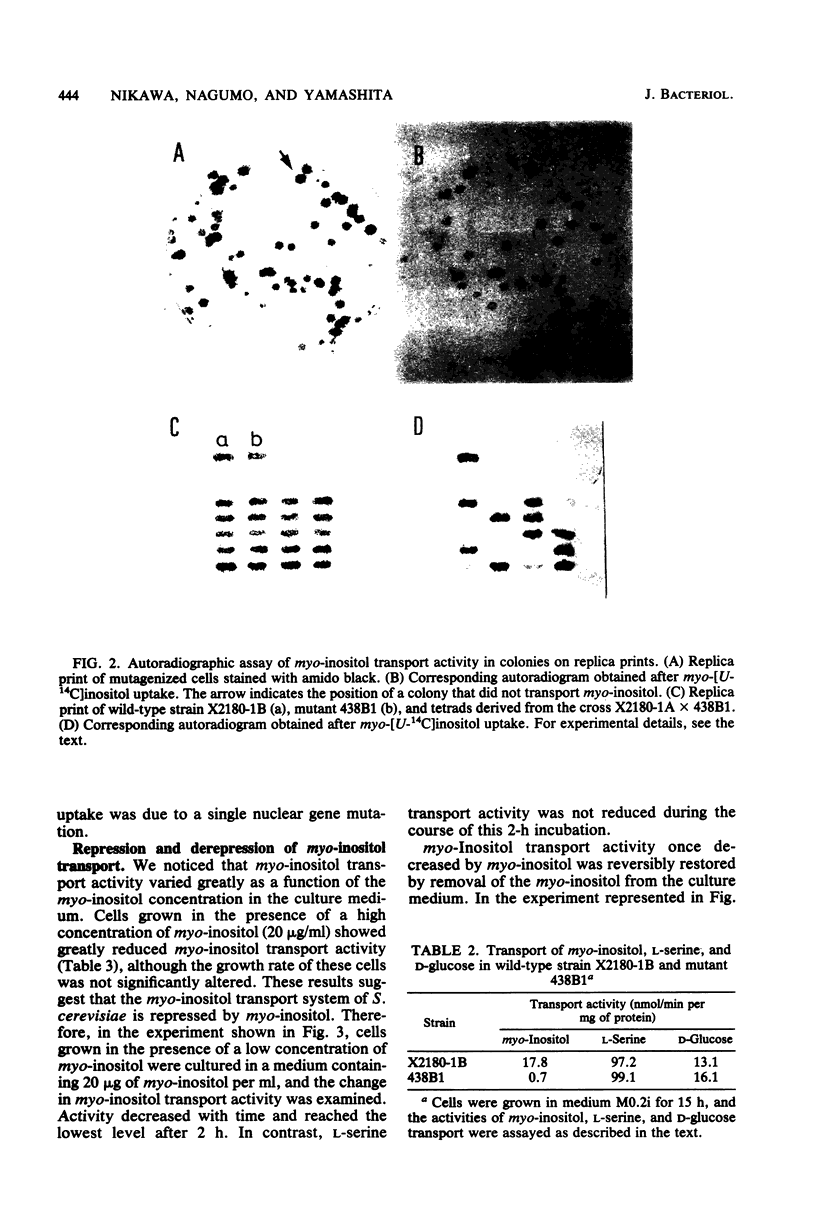
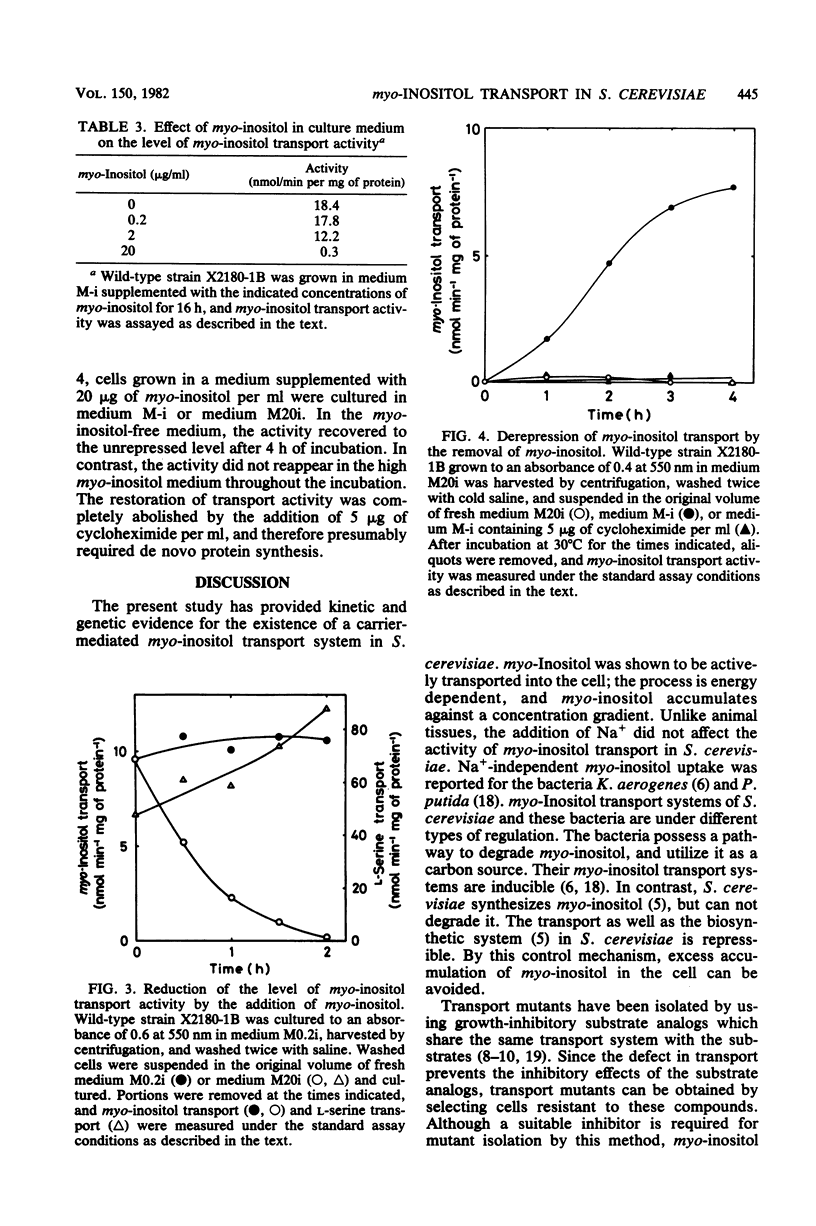
myo-Inositol uptake in Saccharomyces cerevisiae was dependent on temperature, time, and substrate concentration. The transport obeyed saturation kinetics with an apparent Km for myo-inositol of 0.1 mM, myo-Inositol analogs, such as scyllo-inositol, 2-inosose, mannitol, and 1,2-cyclohexanediol, had no effect on myo-inositol uptake, myo-Inositol uptake required metabolic energy. Removal of D-glucose resulted in a loss of activity, and azide and cyanide ions were inhibitory. In the presence of D-glucose, myo-inositol was accumulated in the cells against a concentration gradient. A myo-inositol transport mutant was isolated from UV-mutagenized S. cerevisiae cells using the replica-printing technique. The defect in myo-inositol uptake was due to a single nuclear gene mutation. The activities of L-serine and D-glucose transport were not affected by the mutation. Thus it was shown that S. cerevisiae grown under the present culture conditions possessed a single and specific myo-inositol transport system. myo-Inositol transport activity was reduced by the addition of myo-inositol to the culture medium. The activity was reversibly restored by the removal of myo-inositol from the medium. This restoration of activity was completely abolished by cycloheximide.
Full text
PDF


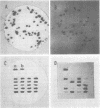
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker G. W., Lester R. L. Biosynthesis of phosphoinositol-containing sphingolipids from phosphatidylinositol by a membrane preparation from Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):747–754. doi: 10.1128/jb.142.3.747-754.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary W. F., Crane R. K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim Biophys Acta. 1970 Apr 21;203(2):308–316. doi: 10.1016/0005-2736(70)90145-8. [DOI] [PubMed] [Google Scholar]
- Cheneval J. P., Deshusses J., Posternak T. Sur le transport de l'inositol chez Schizosaccharomyces pombe. Biochim Biophys Acta. 1970 Apr 21;203(2):348–350. doi: 10.1016/0005-2736(70)90152-5. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Davis R. H. Screening for amino acid pool mutants of Neurospora and yeasts: replica-printing technique. J Bacteriol. 1979 Mar;137(3):1437–1438. doi: 10.1128/jb.137.3.1437-1438.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae: properties of a repressible enzyme system in extracts of wild-type (Ino+) cells. J Bacteriol. 1976 Apr;126(1):232–242. doi: 10.1128/jb.126.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshusses J., Reber G. Myo-inositol transport in Aerobacter aerogenes. Biochim Biophys Acta. 1972 Aug 9;274(2):598–605. doi: 10.1016/0005-2736(72)90206-4. [DOI] [PubMed] [Google Scholar]
- Gits J. J., Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. 3. Evidence for a specific methionine-transporting system. Biochim Biophys Acta. 1967 Jul 3;135(3):507–516. doi: 10.1016/0005-2736(67)90040-5. [DOI] [PubMed] [Google Scholar]
- Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. II. Evidence for a specific lysine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):339–346. doi: 10.1016/0304-4165(66)90388-6. [DOI] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Choline transport in Saccharomyces cerevisiae. J Bacteriol. 1980 Jul;143(1):176–181. doi: 10.1128/jb.143.1.176-181.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Induction of choline transport and its role in the stimulation of the incorporation of choline into phosphatidylcholine by polyamines in a polyamine auxotroph of Saccharomyces cerevisiae. Eur J Biochem. 1981 May;116(1):1–6. doi: 10.1111/j.1432-1033.1981.tb05292.x. [DOI] [PubMed] [Google Scholar]
- Johnstone R. M., Sung C. P. Transport of myo-inositol in Ehrlich ascites cells. Biochim Biophys Acta. 1967;135(5):1052–1055. doi: 10.1016/0005-2736(67)90074-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Raetz C. R. Isolation of Escherichia coli mutants defective in enzymes of membrane lipid synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2274–2278. doi: 10.1073/pnas.72.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rber G., Belet M., Deshusses J. Myo-inositol transport system in Pseudomonas putida. J Bacteriol. 1977 Sep;131(3):872–875. doi: 10.1128/jb.131.3.872-875.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytka J. Positive selection of general amino acid permease mutants in Saccharomyces cerevisiae. J Bacteriol. 1975 Feb;121(2):562–570. doi: 10.1128/jb.121.2.562-570.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Wada E., Tsumita T. myo-Inositol binding and transport in brush border membranes of rat kidney. Biochim Biophys Acta. 1977 Jan 4;464(1):108–117. doi: 10.1016/0005-2736(77)90374-1. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Oshima A. Regulation of phosphatidylethanolamine methyltransferase level by myo-inositol in Saccaromyces cerevisiae. Eur J Biochem. 1980 Mar;104(2):611–616. doi: 10.1111/j.1432-1033.1980.tb04465.x. [DOI] [PubMed] [Google Scholar]